POLR2J2 Mouse Monoclonal Antibody [Clone ID: OTI3G4] Product Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Human Isoform of RNA Polymerase II Subunit Hrpb11bα Specifically Interacts with Transcription Factor ATF4
International Journal of Molecular Sciences Article The Human Isoform of RNA Polymerase II Subunit hRPB11bα Specifically Interacts with Transcription Factor ATF4 Sergey A. Proshkin 1,2, Elena K. Shematorova 1 and George V. Shpakovski 1,* 1 Laboratory of Mechanisms of Gene Expression, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, 117997 Moscow, Russia; [email protected] (S.A.P.); [email protected] (E.K.S.) 2 Engelhardt Institute of Molecular Biology, Center for Precision Genome Editing and Genetic Technologies for Biomedicine, 119991 Moscow, Russia * Correspondence: [email protected]; Tel.: +7-495-3306583; Fax: +7-495-3357103 Received: 25 November 2019; Accepted: 22 December 2019; Published: 24 December 2019 Abstract: Rpb11 subunit of RNA polymerase II of Eukaryotes is related to N-terminal domain of eubacterial α subunit and forms a complex with Rpb3 subunit analogous to prokaryotic α2 homodimer, which is involved in RNA polymerase assembly and promoter recognition. In humans, a POLR2J gene family has been identified that potentially encodes several hRPB11 proteins differing mainly in their short C-terminal regions. The functions of the different human specific isoforms are still mainly unknown. To further characterize the minor human specific isoform of RNA polymerase II subunit hRPB11bα, the only one from hRPB11 (POLR2J) homologues that can replace its yeast counterpart in vivo, we used it as bait in a yeast two-hybrid screening of a human fetal brain cDNA library. By this analysis and subsequent co-purification assay in vitro, we identified transcription factor ATF4 as a prominent partner of the minor RNA polymerase II (RNAP II) subunit hRPB11bα. -

Identification of Key Pathways and Genes in Dementia Via Integrated Bioinformatics Analysis
bioRxiv preprint doi: https://doi.org/10.1101/2021.04.18.440371; this version posted July 19, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Identification of Key Pathways and Genes in Dementia via Integrated Bioinformatics Analysis Basavaraj Vastrad1, Chanabasayya Vastrad*2 1. Department of Biochemistry, Basaveshwar College of Pharmacy, Gadag, Karnataka 582103, India. 2. Biostatistics and Bioinformatics, Chanabasava Nilaya, Bharthinagar, Dharwad 580001, Karnataka, India. * Chanabasayya Vastrad [email protected] Ph: +919480073398 Chanabasava Nilaya, Bharthinagar, Dharwad 580001 , Karanataka, India bioRxiv preprint doi: https://doi.org/10.1101/2021.04.18.440371; this version posted July 19, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract To provide a better understanding of dementia at the molecular level, this study aimed to identify the genes and key pathways associated with dementia by using integrated bioinformatics analysis. Based on the expression profiling by high throughput sequencing dataset GSE153960 derived from the Gene Expression Omnibus (GEO), the differentially expressed genes (DEGs) between patients with dementia and healthy controls were identified. With DEGs, we performed a series of functional enrichment analyses. Then, a protein–protein interaction (PPI) network, modules, miRNA-hub gene regulatory network and TF-hub gene regulatory network was constructed, analyzed and visualized, with which the hub genes miRNAs and TFs nodes were screened out. Finally, validation of hub genes was performed by using receiver operating characteristic curve (ROC) analysis. -

Supplementary Table 1 Genes Tested in Qrt-PCR in Nfpas
Supplementary Table 1 Genes tested in qRT-PCR in NFPAs Gene Bank accession Gene Description number ABI assay ID a disintegrin-like and metalloprotease with thrombospondin type 1 motif 7 ADAMTS7 NM_014272.3 Hs00276223_m1 Rho guanine nucleotide exchange factor (GEF) 3 ARHGEF3 NM_019555.1 Hs00219609_m1 BCL2-associated X protein BAX NM_004324 House design Bcl-2 binding component 3 BBC3 NM_014417.2 Hs00248075_m1 B-cell CLL/lymphoma 2 BCL2 NM_000633 House design Bone morphogenetic protein 7 BMP7 NM_001719.1 Hs00233476_m1 CCAAT/enhancer binding protein (C/EBP), alpha CEBPA NM_004364.2 Hs00269972_s1 coxsackie virus and adenovirus receptor CXADR NM_001338.3 Hs00154661_m1 Homo sapiens Dicer1, Dcr-1 homolog (Drosophila) (DICER1) DICER1 NM_177438.1 Hs00229023_m1 Homo sapiens dystonin DST NM_015548.2 Hs00156137_m1 fms-related tyrosine kinase 3 FLT3 NM_004119.1 Hs00174690_m1 glutamate receptor, ionotropic, N-methyl D-aspartate 1 GRIN1 NM_000832.4 Hs00609557_m1 high-mobility group box 1 HMGB1 NM_002128.3 Hs01923466_g1 heterogeneous nuclear ribonucleoprotein U HNRPU NM_004501.3 Hs00244919_m1 insulin-like growth factor binding protein 5 IGFBP5 NM_000599.2 Hs00181213_m1 latent transforming growth factor beta binding protein 4 LTBP4 NM_001042544.1 Hs00186025_m1 microtubule-associated protein 1 light chain 3 beta MAP1LC3B NM_022818.3 Hs00797944_s1 matrix metallopeptidase 17 MMP17 NM_016155.4 Hs01108847_m1 myosin VA MYO5A NM_000259.1 Hs00165309_m1 Homo sapiens nuclear factor (erythroid-derived 2)-like 1 NFE2L1 NM_003204.1 Hs00231457_m1 oxoglutarate (alpha-ketoglutarate) -

WO 2012/174282 A2 20 December 2012 (20.12.2012) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2012/174282 A2 20 December 2012 (20.12.2012) P O P C T (51) International Patent Classification: David [US/US]; 13539 N . 95th Way, Scottsdale, AZ C12Q 1/68 (2006.01) 85260 (US). (21) International Application Number: (74) Agent: AKHAVAN, Ramin; Caris Science, Inc., 6655 N . PCT/US20 12/0425 19 Macarthur Blvd., Irving, TX 75039 (US). (22) International Filing Date: (81) Designated States (unless otherwise indicated, for every 14 June 2012 (14.06.2012) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, English (25) Filing Language: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, Publication Language: English DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, (30) Priority Data: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 61/497,895 16 June 201 1 (16.06.201 1) US MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, 61/499,138 20 June 201 1 (20.06.201 1) US OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, 61/501,680 27 June 201 1 (27.06.201 1) u s SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, 61/506,019 8 July 201 1(08.07.201 1) u s TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Beyond Gene Expression
Beyond gene expression Citation for published version (APA): Gupta, R. (2021). Beyond gene expression: novel methods and applications of transcript expression analyses in RNA-Seq. Maastricht University. https://doi.org/10.26481/dis.20210304rg Document status and date: Published: 01/01/2021 DOI: 10.26481/dis.20210304rg Document Version: Publisher's PDF, also known as Version of record Please check the document version of this publication: • A submitted manuscript is the version of the article upon submission and before peer-review. There can be important differences between the submitted version and the official published version of record. People interested in the research are advised to contact the author for the final version of the publication, or visit the DOI to the publisher's website. • The final author version and the galley proof are versions of the publication after peer review. • The final published version features the final layout of the paper including the volume, issue and page numbers. Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal. -

Termination of RNA Polymerase II Transcription by the 5’-3’ Exonuclease Xrn2
TERMINATION OF RNA POLYMERASE II TRANSCRIPTION BY THE 5’-3’ EXONUCLEASE XRN2 by MICHAEL ANDRES CORTAZAR OSORIO B.S., Universidad del Valle – Colombia, 2011 A thesis submitted to the Faculty of the Graduate School of the University of Colorado in partial fulfillment of the requirements for the degree of Doctor of Philosophy Molecular Biology Program 2018 This thesis for the Doctor of Philosophy degree by Michael Andrés Cortázar Osorio has been approved for the Molecular Biology Program by Mair Churchill, Chair Richard Davis Jay Hesselberth Thomas Blumenthal James Goodrich David Bentley, Advisor Date: Aug 17, 2018 ii Cortázar Osorio, Michael Andrés (Ph.D., Molecular Biology) Termination of RNA polymerase II transcription by the 5’-3’ exonuclease Xrn2 Thesis directed by Professor David L. Bentley ABSTRACT Termination of transcription occurs when RNA polymerase (pol) II dissociates from the DNA template and releases a newly-made mRNA molecule. Interestingly, an active debate fueled by conflicting reports over the last three decades is still open on which of the two main models of termination of RNA polymerase II transcription does in fact operate at 3’ ends of genes. The torpedo model indicates that the 5’-3’ exonuclease Xrn2 targets the nascent transcript for degradation after cleavage at the polyA site and chases pol II for termination. In contrast, the allosteric model asserts that transcription through the polyA signal induces a conformational change of the elongation complex and converts it into a termination-competent complex. In this thesis, I propose a unified allosteric-torpedo mechanism. Consistent with a polyA site-dependent conformational change of the elongation complex, I found that pol II transitions at the polyA site into a mode of slow transcription elongation that is accompanied by loss of Spt5 phosphorylation in the elongation complex. -

Biomedical Robots. Application to Translational Medicine
Biomedical robots. Application to translational medicine. Enrique J. deAndrés-Galiana Supervisors: Prof. Juan Luis Fernández-Martínez & Prof. Oscar Luaces This dissertation is submitted under the PhD program of Mathematics and Statistics May 2016 RESUMEN DEL CONTENIDO DE TESIS DOCTORAL 1.- Título de la Tesis Español/Otro Idioma: Inglés: Diseño de robots biomédicos. Aplicaciones en Biomedical robots. Application to translational medicina traslacional. medicine. 2.- Autor Nombre: Enrique Juan de Andrés Galiana DNI/Pasaporte/NIE: Programa de Doctorado: Matemáticas y Estadística. Órgano responsable: Departamento de Matemáticas. RESUMEN (en español) Esta tesis trata sobre el análisis y diseño de robots biomédicos y su aplicación a la medicina traslacional. Se define un robot biomédico como el conjunto de técnicas provenientes de la matemática aplicada, estadística y ciencias de la computación capaces de analizar datos biomédicos de alta dimensionalidad, aprender dinámicamente de dichos datos, extraer nuevo BIS - conocimiento e hipótesis de trabajo, y finalmente realizar predicciones con su incertidumbre asociada, cara a la toma de decisiones biomédicas. Se diseñan y analizan diferentes algorit- 010 - mos de aprendizaje, de reducción de la dimensión y selección de atributos, así como técnicas de optimización global, técnicas de agrupamiento no supervisado, clasificación y análisis de VOA incertidumbre. Dichas metodologías se aplican a datos a pie de hospital y de expresión génica - en predicción de fenotipos para optimización del diagnóstico, pronóstico, tratamiento y análisis de toxicidades. MAT - Se muestra que es posible establecer de modo sencillo el poder discriminatorio de las variables FOR pronóstico, y que dichos problemas de clasificación se aproximan a un comportamiento linealmente separable cuando se reduce la dimensión al conjunto de variables principales que definen el alfabeto del problema biomédico y están por tanto relacionadas con su génesis. -

1 Novel Expression Signatures Identified by Transcriptional Analysis
ARD Online First, published on October 7, 2009 as 10.1136/ard.2009.108043 Ann Rheum Dis: first published as 10.1136/ard.2009.108043 on 7 October 2009. Downloaded from Novel expression signatures identified by transcriptional analysis of separated leukocyte subsets in SLE and vasculitis 1Paul A Lyons, 1Eoin F McKinney, 1Tim F Rayner, 1Alexander Hatton, 1Hayley B Woffendin, 1Maria Koukoulaki, 2Thomas C Freeman, 1David RW Jayne, 1Afzal N Chaudhry, and 1Kenneth GC Smith. 1Cambridge Institute for Medical Research and Department of Medicine, Addenbrooke’s Hospital, Hills Road, Cambridge, CB2 0XY, UK 2Roslin Institute, University of Edinburgh, Roslin, Midlothian, EH25 9PS, UK Correspondence should be addressed to Dr Paul Lyons or Prof Kenneth Smith, Department of Medicine, Cambridge Institute for Medical Research, Addenbrooke’s Hospital, Hills Road, Cambridge, CB2 0XY, UK. Telephone: +44 1223 762642, Fax: +44 1223 762640, E-mail: [email protected] or [email protected] Key words: Gene expression, autoimmune disease, SLE, vasculitis Word count: 2,906 The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Annals of the Rheumatic Diseases and any other BMJPGL products to exploit all subsidiary rights, as set out in their licence (http://ard.bmj.com/ifora/licence.pdf). http://ard.bmj.com/ on September 29, 2021 by guest. Protected copyright. 1 Copyright Article author (or their employer) 2009. -

Méthylations De L'histone H3 Et Contrôle Épigénétique Des
M´ethylations de l'histone H3 et contr^ole´epig´en´etique des propri´et´esdes cellules souches de gliomes Alexandra Bogeas To cite this version: Alexandra Bogeas. M´ethylations de l'histone H3 et contr^ole´epig´en´etiquedes propri´et´esdes cellules souches de gliomes. M´edecinehumaine et pathologie. Universit´eRen´eDescartes - Paris V, 2013. Fran¸cais. <NNT : 2013PA05P620>. <tel-01170633> HAL Id: tel-01170633 https://tel.archives-ouvertes.fr/tel-01170633 Submitted on 2 Jul 2015 HAL is a multi-disciplinary open access L'archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destin´eeau d´ep^otet `ala diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publi´esou non, lished or not. The documents may come from ´emanant des ´etablissements d'enseignement et de teaching and research institutions in France or recherche fran¸caisou ´etrangers,des laboratoires abroad, or from public or private research centers. publics ou priv´es. Université Paris Descartes PARIS V Ecole Doctorale MTCE «Médicament, Toxicologie, Chimie et Environnement» THÈSE de DOCTORAT de l’UNIVERSITE PARIS V Spécialité : Neurosciences En vue de l’obtention du grade de Docteur de l’Université Paris V Présentée par Alexandra BOGEAS Méthylations de l’histone H3 et contrôle épigénétique des propriétés des cellules souches de gliomes Thèse dirigée par le Dr Hervé CHNEIWEISS Soutenue le 29 Novembre 2013 Devant le Jury composé de : Madame le Docteur Sylvie ROBINE Président Monsieur le Professeur -

POLR2J2 Recombinant Protein Description Product Info
9853 Pacific Heights Blvd. Suite D. San Diego, CA 92121, USA Tel: 858-263-4982 Email: [email protected] 32-2699: POLR2J2 Recombinant Protein Alternative Name : HRPB11B,RPB11b1,POLR2J2,DNA-directed RNA polymerase II subunit RPB11-b1. Description Source : E.coli. POLR2J2 Human Recombinant produced in E.coli is a single, non-glycosylated polypeptide chain containing 138 amino acids (1-115) and having a molecular mass of 15.5kDa. POLR2J2 is fused to a 23 amino acid His-tag at N-terminus & purified by proprietary chromatographic techniques. POLR2J2 belongs to the RNA polymerase II subunit 11 gene family, that includes 3 genes in a cluster on chromosome 7q22.1 and a pseudogene on chromosome 7p13. DNA directed RNA polymerase II polypeptide J family encodes a subunit of RNA polymerase II, the polymerase which is responsible for synthesizing messenger RNA in eukaryotes. This locus produces multiple, otherwise spliced transcripts which express isoforms with distinct C-termini compared to DNA directed RNA polymerase II polypeptide J. Most or all variants are spliced to include additional non- coding exons at the 3' end that makes them candidates for nonsense-mediated decay (NMD). Therefore, it is unknown if this locus expresses a protein or proteins in vivo. Product Info Amount : 10 µg Purification : Greater than 95% as determined by SDS-PAGE. The POLR2J2 solution (0.25mg/ml) contains 20mM Tris-HCl buffer (pH 8.0), 0.15M NaCl, 10% Content : glycerol, 1mM DTT and 250mM Imidazole. Store at 4°C if entire vial will be used within 2-4 weeks. Store, frozen at -20°C for longer periods of Storage condition : time. -
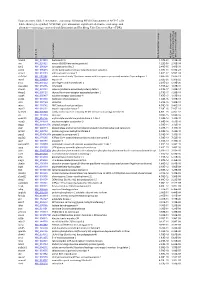
Supplementary Table 3. Alternative Exon Usage Following PRMT6 Knockdown in MCF-7 Cells. Table Shows Gene Symbol, NCBI Link
Supplementary Table 3. Alternative exon usage following PRMT6 knockdown in MCF-7 cells. Table shows gene symbol, NCBI link, gene annotation, significant alternative exon usage and alternative exon usage corrected with Benjamini and Hochberg False Discovery Rate (FDR). Gene Symbol NCBILink Annotation Gene Alt. Exon Usage Significant Alt. Exon Usage corrected with Benjamini and Hochberg FalseDiscovery Rate (FDR) hmcn1 NM_031935 hemicentin 1 1.37E-38 1.18E-34 nin NM_020921 ninein (GSK3B interacting protein) 1.22E-38 2.10E-34 sytl2 NM_206928 synaptotagmin-like 2 2.48E-35 1.43E-31 sptlc1 NM_006415 serine palmitoyltransferase long chain base subunit 1 1.37E-31 5.88E-28 antxr1 NM_018153 anthrax toxin receptor 1 1.62E-27 5.59E-24 slc7a5p1 NR_002593 solute carrier family 7 (cationic amino acid transporter y+ system) member 5 pseudogene 1 2.66E-26 7.63E-23 myo6 NM_004999 myosin VI 2.52E-25 6.20E-22 zhx1 NM_007222 zinc fingers and homeoboxes 1 2.41E-23 5.19E-20 kiaa1429 NM_015496 KIAA1429 8.71E-22 1.67E-18 ctnnd1 NM_001331 catenin (cadherin-associated protein) delta 1 2.34E-17 4.03E-14 thrap1 NM_005121 thyroid hormone receptor associated protein 1 2.73E-17 4.28E-14 ncoa4 NM_005437 nuclear receptor coactivator 4 7.87E-17 1.13E-13 dock1 NM_001380 dedicator of cytokinesis 1 1.04E-16 1.38E-13 utrn NM_007124 utrophin 1.21E-16 1.48E-13 mina NM_153182 MYC induced nuclear antigen 4.74E-16 5.44E-13 myef2 NM_016132 myelin expression factor 2 7.29E-16 7.85E-13 fam62b NM_020728 family with sequence similarity 62 (C2 domain containing) member B 4.67E-15 4.74E-12 -

Chromosomal Breakpoints in Primary Colon Cancer Cluster at Sites of Structural Variants in the Genome
Research Article Chromosomal Breakpoints in Primary Colon Cancer Cluster at Sites of Structural Variants in the Genome Jordi Camps,1 Marian Grade,1,3 Quang Tri Nguyen,1 Patrick Ho¨rmann,1 Sandra Becker,1 Amanda B. Hummon,1 Virginia Rodriguez,2 Settara Chandrasekharappa,2 Yidong Chen,1 Michael J. Difilippantonio,1 Heinz Becker,3 B. Michael Ghadimi,3 and Thomas Ried1 1Genetics Branch, Center for Cancer Research, National Cancer Institute/NIH; 2Genome Technology Branch, National Human Genome Research Institute/NIH, Bethesda, Maryland; and 3Department of General and Visceral Surgery, University Medicine Go¨ttingen, Go¨ttingen, Germany Abstract 8q, 13, and 20q as well as losses of chromosomes 4q, 8p, 17p, and 18q (2). Genomic aberrations on chromosome 8 are common in colon cancer, and are associated with lymph node and distant Within the last decade, microarray technology has been metastases as well as with disease susceptibility. This extensively applied to survey the cellular transcriptome of common prompted us to generate a high-resolution map of genomic solid tumors, including colorectal cancer, and for colon cancers, imbalances of chromosome 8 in 51 primary colon carcinomas gene expression signatures were subsequently correlated with using a custom-designed genomic array consisting of a tiling clinical outcome (for reviews, see refs. 3–5). However, high- path of BAC clones. This analysis confirmed the dominant role resolution mapping of chromosomal copy number changes has of this chromosome. Unexpectedly, the position of the break- only recently been achieved using BAC or cDNA clone-based arrays points suggested colocalization with structural variants in the (6–10).