Oak Willow Cherry, Plum Blueberry, Cranberry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Technical Note 30: Ability of Pacific Northwest Shrubs to Root From
TECHNICAL NOTES _____________________________________________________________________________________________ U.S. DEPT. OF AGRICULTURE NATURAL RESOURCES CONSERVATION SERVICE PORTLAND, OREGON DATE: September 2002 PLANT MATERIALS TECHNICAL NOTE NO. 30 Ability of Pacific Northwest Native Shrubs to Root from Hardwood Cuttings (with Summary of Propagation Methods for 22 Species) Dale C. Darris Conservation Agronomist Corvallis Plant Materials Center SUMMARY There is a need for additional information on how well native shrubs root from hardwood cuttings. First, nurseries are seeking to refine vegetative propagation techniques. Second, practitioners in the fields of riparian/wetland restoration and streambank stabilization are looking for species that are easy to root from unrooted dormant material that will provide cost competitive alternatives to native willows (Salix spp.) and redosier dogwood (Cornus sericea) for direct sticking of cuttings and soil bioengineering practices. The purpose of this work was to screen 15 shrubs indigenous to the Pacific Northwest USA for their ability to root from hardwood cuttings with and without a rooting compound in a greenhouse mist bench, well drained field, nursery bed, and saturated substrate (artificial pond). If a species roots easily, it has greater potential for planting as dormant cuttings, live stakes, or whips directly on a revegetation site, and may warrant further evaluation for fascines, brush mattresses, or other soil bioengineering practices. Based on the rooting ability of hardwood cuttings made from 1 (or 2) year old wood, those species with good potential include black twinberry (Lonicera involucrata), salmonberry (Rubus spectabilis), common snowberry (Symphoricarpos albus), and Pacific ninebark (Physocarpus capitatus). Coyote brush (Baccharis pilularis) and red elderberry (Sambucus racemosa) have fair potential. Indian plum (Oemlaria cerasiformis), mock orange (Philadelphus lewisii), and red flowering currant (Ribes sanguineum) may apply under limited circumstances, select uses, or ideal conditions. -

Salt-Spray Tolerant Groundcovers, Shrubs, and Trees for Eastern Long Island
Salt-spray Tolerant Groundcovers, Shrubs, and Trees for Eastern Long Island Aerial salts carried by on-shore breezes, fog, and wind can injure plants sensitive to salt deposition. The plants listed below have displayed either high or moderate tolerance to salt spray. Most species listed have displayed high tolerance ( little to no damage). Those species noted as having moderate tolerance may show signs of salt injury (tip dieback, foliar damage, reduced growth). Moderately-tolerant species should be planted in areas away from direct salt-spray exposure. Salt-spray tolerance applies to aerial deicing salts as well. The plants listed were chosen because they are relatively disease and pest resistance (unless noted), and well-suited for eastern Long Island. Perennial Groundcovers Foliage Common Name Scientific Name Habit & Comments* Deciduous Beach Pea Lathyrus maritimus native, flowering, silver-green foliage Evergreen Bearberry Arctostaphylos uva-ursi native, low-growing, soft stems Evergreen Shore Juniper Juniperus conferta spreading, dense Semi-evergreen Willowleaf Cotoneaster Cotoneaster salicifolius weeping, cascading Semi-evergreen Lily Turf Liriope muscari grass-like appearance Woody Shrubs (fifteen feet or less in height) Deciduous False Indigo-bush Amorpha fruticosa flowering shrub, prefers wet sites Deciduous Red Chokeberry Aronia arbutifolia moderate salt tolerance, native, early flowering Deciduous Black Chokecherry Aronia melanocarpa moderate salt tolerance, native, early flowering Deciduous Eastern Baccharis Baccharis halimifolia native, tolerates wet sites Deciduous Sweet Pepperbush Clethra alnifolia native, flowers late Deciduous Sweetfern Comptonia peregrina native, prefers dry sites Deciduous Creeping Cotoneaster Cotoneaster adpressus mounded, spreading Deciduous Cotoneaster Cotoneaster divaricatus upright, arching habit Deciduous Forsythia Forsythia x intermedia early spring flowering Deciduous Common Witchhazel Hamamelis virginiana native, very early flowering Deciduous Rose of Sharon Hibuscus syriacus moderately salt tolerant, flowering, mod. -

Genetic and Phenotypic Characterization of Figured Wood in Poplar
Genetic and Phenotypic Characterization of Figured Wood in Poplar Youran Fan1,2, Keith Woeste1,2, Daniel Cassens1, Charles Michler1,2, Daniel Szymanski3, and Richard Meilan1,2 1Department of Forestry and Natural Resources, 2Hardwood Tree Improvement and Regeneration Center, and 3Department of Agronomy; Purdue University, West Lafayette, Indiana 47907 Abstract Materials and Methods When “Curly Aspen” (Populus canescens) was first Preliminary Results characterized in the early 1940’s[1], it attracted the attention from the wood-products industry because Genetically engineer commercially 1) Histological sections reveal that “Curly Aspen” has strong “Curly Aspen” produces an attractive veneer as a important trees to form figure. ray flecks (Fig. 10) but this is not likely to be responsible result of its figured wood. Birdseye, fiddleback and for the figure seen. quilt are other examples of figured wood that are 2) Of the 15 SSR primer pairs[6, 7, 8] tested, three have been commercially important[2]. These unusual grain shown to be polymorphic. Others are now being tested. patterns result from changes in cell orientation in Figure 6. Pollen collection. Branches of Figure 7. Pollination. Branches Ultimately, our genetic fingerprinting technique will allow “Curly Aspen” were “forced” to shed collected from a female P. alba us to distinguish “Curly Aspen” from other genotypes. the xylem. Although 50 years have passed since Figure 1. Birdseye in maple. pollen under controlled conditions. growing at Iowa State University’s finding “Curly Aspen”, there is still some question Rotary cut, three-piece book McNay Farm (south of Lucas, IA). 3) 17 jars of female P. alba branches have been pollinated match (origin: North America). -

Seeds of Oxyccocus Palustris Pers. from Ericaceae Family
Biology of germination of medicinal plant seeds. Part XXIII: Seeds of Oxyccocus palustris Pers. from Ericaceae family WALDEMAR BUCHWALD*, JAN KOZŁOWSKI, ELŻBIETA BILIŃSKA The Branch of Medicinal Plants of the Institute of Natural Fibres and Medicinal Plants Libelta 27 61-707 Poznań, Poland *corresponding author: [email protected], phone: +4861 6517190, fax: +4861 6517192 S u m m a r y At the beginning a short characteristic of Ericaceae family is elaborated then the biology of seeds germination of Oxyccocus palustris Pers. is described. In this experiments, the opti- mal condition of ability of germination of Oxyccocus palustris Pers. was established. It was found that the light and variable temperature (30oC over 8 hours, 20oC over 16 hours) were optimal for analysis of Oxyccocus palustris seed germination ability. The results also show that the pre-sowing treatments (stratification) were significant to obtain a higher percent of germinating seeds in comparison to non-stratified seeds. The capacity of germination of Oxyccocus palustris Pers. seeds stored in unheated room conditions gradually decreased in the first year after harvest. The viability of Oxyccocus palustris seeds is very short. Three years after harvest time, the seeds do not germinate. Key words: Ericaceae family, Oxyccocus palustris Pers., germination, seeds INTRODUCTION The characteristics of Ericaceae family In Poland 15 species from 10 genera belonging to Ericaceae family are noticed [1-5]. It is relatively few taxa in comparison to 82 genera and to over 2,500 species widespread all over the world. The plants appear as a dwarf shrub, sub-shrub, shrub or a small tree, usually with alternating leathery and often evergreen leaves. -

Quercus Phellos.Indd
Quercus phellos (Willow Oak) Beech Family (Fagaceae) Introduction: Willow oak is a member of the red oak group with willow-shaped leaves. The fi ne foliage of the willow oak is one of its best ornamental features. The willow oak has excellent texture, rounded form, attrac- tive bark, and beautiful winter features (fi ne-texture, persistent leaves and twiggy form). Culture: The willow oak is an excellent choice as a shade tree. It thrives in moist, well-drained, acidic soil and full sun. The willow oak will tolerate pollution and drought and is considered a trouble-free tree as long as soil pH is acidic. Willow oak has a fi brous root system and is therefore easy to transplant. It has no serious disease or Botanical Characteristics: insect problems. As little as 1 inch of fi ll soil can kill an oak. Native habitat: Southeastern USA in alluvial soils, swamps. Additional comments: Willow oak is an excellent large shade tree. Its Growth habit: Unique among the oaks, the wil- low oak has a rounded habit. fi ne texture contrasts with the coarseness of most other red oaks. It is one of the best oaks for avenue plantings Tree size: Growing fast for an oak, it can reach or large residences. Willow oak is a fast-growing oak 70’ in height and equal width. that transplants easily and is tolerant of a wide range of growing conditions. Flower and fruit: Female fl owers are incon- Willow oak is a member of the red oak group spicuous; however the pendulous male catkins without lobed leaves. -
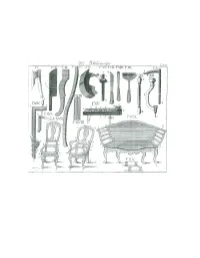
Tools of the Cabinetmaker, but Also Like the Cartwright, the Hatchet (Handbeil) and the Drawknife (Schneidemesser)
CHAPTER FIVE The Chairmaker The chairmaker bears the name in common with English chairmakers presumably because his trade is originally transplanted from England to Germany, or because several types of chairs that are made in his workshop have been common first in England. In the making of chairs, the settee (Canape), and sofa, he wields not only the plane and other tools of the cabinetmaker, but also like the cartwright, the hatchet (Handbeil) and the drawknife (Schneidemesser). I. In most regions, and especially in the German coastal cities, chairmakers make their chairs out of red beech wood, in Magdeburg out of linden wood, and in Berlin out of serviceberry wood (Elsenholz). Red beech is lacking in our area, and the cabinetmaker, who before the arrival to Berlin of chairmakers that made wooden chair frames, chose therefore serviceberry wood in place of red beech. Likewise the chairmakers, when they arrived in Berlin, found that circumstances also compelled them to build their chairs out of serviceberry wood. If the customer explicitly requires it, and will pay especially for it, they sometimes build chairs out of walnut, plum wood, pearwood, and mahogany wood, and for very distinguished and wealthy persons out of cedarwood. The chairmaker obtains the serviceberry wood partly in boards that are one to five inches thick and partly in logs. The farmer in the [town of] Mark Brandenburg brings this wood, partly in logs and also in boards, to Berlin to sell, but the strongest and best comes from Poland. If the wood has not sufficiently dried when purchased by the chairmaker it must stay some time longer and properly dry. -

Verticillium Wilt of Fraxinus Excelsior
Verticillium wilt of Fraxinus excelsior - ' . ; Jt ""* f- "" UB-^/^'IJ::J CENTRALE LANDBOUWCATALOGUS 0000 0611 8323 locjs Promotoren: Dr.Ir. R.A.A. Oldeman Hoogleraar ind e Bosteelt en Bosoecologie Dr.Ir. J. Dekker Emeritus Hoogleraar ind e Fytopathologie /OMOS-Zöl,^ Jelle A. Hiemstra Verticillium wilt of Fraxinus excelsior Proefschrift ter verkrijging van de graad van doctor in de landbouw- en milieuwetenschappen, op gezag van de rector magnificus, dr. C.M. Karssen, inhe t openbaar te verdedigen op dinsdag 18apri l 1995 des namiddags omvie r uur ind e aula van de Landbouwuniversiteit te Wageningen J\ ABSTRACT Hiemstra, J.A. (1995). Verticillium wilt of Fraxinus excelsior. PhD Thesis, Wageningen Agricultural University, The Netherlands, xvi + 213 pp, 40 figs., 28 tables, 4 plates with colour pictures, 327 refs., English and Dutch summaries. ISBN 90-5485-360-3 Research on ash wilt disease, a common disease of Fraxinus excelsior L. in young forest and landscape plantings in several parts of the Netherlands, is described. By means of a survey for pathogenic fungi in affected trees, inoculation and reisolation experimentsi ti sdemonstrate d thatth ediseas ei scause db y Verticilliumdahliae Kleb . Hostspecificit y andvirulenc e of aV. dahliae isolatefro m ashar ecompare d tothos e of isolatesfro m elm,mapl ean dpotato .Diseas eincidenc ean dprogress , andrecover y of infected trees are investigated through monitoring experiments in two permanent plots in seriously affected forest stands. Monitoring results are related to the results of an aerial survey for ash wilt disease in the province of Flevoland to assess the impact of the disease on ash forests. -

Evergreen Trees Agonis Flexuosa
Evergreen Trees Agonis flexuosa – Peppermint Willow Graceful willow-like evergreen tree (but without the willows voracious root system) with reddish-brown, deeply furrowed bark to 25’-30’. New leaves and twigs have an attractive reddish cast; clustered small white flowers and brownish fruits are not particularly ornamental. Casaurina stricta – Beefwood Pendulous gray branches; resembles a pine somewhat; tolerates drought, heat, wind, fog. Growth to 20’- 30’. Cinnamomum camphora - Camphor Evergreen trees to 40 feet, with 20-foot spread.. In winter foliage is a shiny yellow green. In early spring new foliage may be pink, red or bronze, depending on tree. Unusually strong structure. Clusters of tiny, fragrant yellow flowers in profusion in May. Geijera parviflora- Australian Willow Evergreen trees with graceful, fine-textured leaves, to 30 feet, 20 feet wide. Main branches weep up and out; little branches hang down. Much of the grace of a willow, much of the toughness of eucalyptus, moderate growth and deep non-invasive roots. Laurus nobilis – Grecian Laurel Slow growth 12-40’. Natural habit is compact, broad-based, often multi-stemmed, gradually tapering cone. Leaves lethery, aromatic. Clusters of small yellow flowers followed by black or purple berries. Magnolia Grandiflora – ‘Little Gem’- Dwarf Southern Magnolia Small tree to 20’ in height. Showy white flowers in the summer. Green glossy leaves. Maytenous boaria - Mayten Evergreen tree with slow to moderate growth to an eventual 30-50 feet, with a 15-foot spread, with long and pendulous branchlets hanging down from branches, giving tree a graceful look. Habit and leaves somewhat like a small scale weeping willow. -

Invasive Trees of Georgia Pub10-14
Pub. No. 39 October 2016 Invasive Trees of Georgia by Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia Georgia has many species of trees. Some are native trees and some have been introduced from outside the state, nation, or continent. Most of Georgia’s trees are well- behaved and easily develop into sustainable shade and street trees. A few tree species have an extrodinary ability to upsurp resources and take over sites from other plants. These trees are called invasive because they effectively invade sites, many times eliminat- ing other species of plants. There are a few tree species native to Georgia which are considered invasive in other parts of the country. These native invasives, may be well-behaved in Georgia, but reproduce and take over sites elsewhere, and so have gained an invasive status from at least one other invasive species list. Table 1. There are hundreds of trees which have been introduced to Georgia landscapes. Some of these exotic / naturalized trees are considered invasive. The selected list of Georgia invasive trees listed here are notorious for growing rampantly and being difficult to eradicate. Table 2. Table 1: Native trees considered invasive in other parts of the country. scientific name common name scientific name common name Acacia farnesiana sweet acacia Myrica cerifera Southern bayberry Acer negundo boxelder Pinus taeda loblolly pine Acer rubrum red maple Populus deltoides Eastern cottonwood Fraxinus americana white ash Prunus serotina black cherry Fraxinus pennsylvanica green ash Robinia pseudoacacia black locust Gleditsia triacanthos honeylocust Toxicodendron vernix poison sumac Juniperus virginiana eastern redcedar The University of Georgia is committed to principles of equal opportunity and affirmative action. -

Ericaceae Five Petals, Sometimes Free, Though Usually the Heather Family Fused Together to Form a Tube, Bell Or Urn
RHS GENEALOGY FOR GARDENERS EUDICOTS sepals, free or fused at the base, and four or Ericaceae five petals, sometimes free, though usually The heather family fused together to form a tube, bell or urn. The stamens are in whorls of four or five and Hugely useful in the garden, this family of mainly woody plants includes the heathers the pollen is released from the anthers by (Calluna, Erica, Daboecia), azaleas, rhododendrons, wintergreens (Gaultheria), way of pores at the tips. Pieris and mountain laurels (Kalmia). Commercially significant crops include Fruit blueberries and cranberries (Vaccinium). Fruits are typically dry capsules, though fleshy Size Origins fruits, such as blueberries, are not uncommon. One of the larger families, the Ericaceae contains Earliest evidence of this family dates to the Late Leaves over 3,850 species. Within this great diversity are Cretaceous (about 90 million years ago). Fossils many small genera with one or two species, and suggest that Ericaceae was once more diverse in Most Ericaceae have evergreen, alternate leaves and Vaccinium corymbosum, three titans; Rhododendron (with 1,000 species), Europe, which was home to genera now restricted no stipules. Some species, such as many azaleas, highbush blueberry Erica (850 species) and Vaccinium (500 species). to Asia and/or America. are deciduous, while opposite and whorled leaf It should be noted that azaleas are included arrangements are also known. Leaf margins are within Rhododendron. Flowers entire, toothed or curled under, and some species USES FOR THIS FAMILY (including many rhododendrons) have dense hair Great floral diversity is encompassed by this or scales on the lower surfaces. -

Comparison of Oak and Sugar Maple Distribution and Regeneration in Central Illinois Upland Oak Forests
COmparisON OF OaK AND Sugar MAPLE DistriBUTION AND REGENEratiON IN CEntral ILLINOIS UPLAND OaK FOREsts Peter J. Frey and Scott J. Meiners1 Abstract.—Changes in disturbance frequencies, habitat fragmentation, and other biotic pressures are allowing sugar maple (Acer saccharum) to displace oak (Quercus spp.) in the upland forest understory. The displacement of oaks by sugar maples represents a major management concern throughout the region. We collected seedling microhabitat data from five upland oak forest sites in central Illinois, each differing in age class or silvicultural treatment to determine whether oaks and maples differed in their microhabitat responses to environmental changes. Maples were overall more prevalent in mesic slope and aspect positions. Oaks were associated with lower stand basal area. Both oaks and maples showed significant habitat partitioning, and environmental relationships were consistent across sites. Results suggest that management intensity for oak in upland forests could be based on landscape position. Maple expansion may be reduced by concentrating mechanical treatments in expected areas of maple colonization, while using prescribed fire throughout stands to promote oak regeneration. INTRODUCTION Historically, white oak (Quercus alba) dominated much of the midwestern and eastern U.S. hardwood forests (Abrams and Nowacki 1992, Franklin et al. 1993). Oak is classified as an early successional forest species, and many researchers agree that oak populations were maintained by Native American or lightning-initiated fires (Abrams 2003, Abrams and Nowacki 1992, Hutchinson et al. 2008, Moser et al. 2006, Nowacki and Abrams 2008, Ruffner and Groninger 2006, Shumway et al. 2001). These periodic low to moderate surface fires favored the ecophysiological attributes of oak over those of fire-sensitive, shade-tolerant tree species, thereby continually resetting succession and allowing oaks and other shade-intolerant species to persist in both the canopy and understory (Abrams 2003, Abrams and Nowacki 1992, Crow 1988, Franklin et al. -

Diversity of Fungal Assemblages in Roots of Ericaceae in Two
Diversity of fungal assemblages in roots of Ericaceae in two Mediterranean contrasting ecosystems Ahlam Hamim, Lucie Miche, Ahmed Douaik, Rachid Mrabet, Ahmed Ouhammou, Robin Duponnois, Mohamed Hafidi To cite this version: Ahlam Hamim, Lucie Miche, Ahmed Douaik, Rachid Mrabet, Ahmed Ouhammou, et al.. Diversity of fungal assemblages in roots of Ericaceae in two Mediterranean contrasting ecosystems. Comptes Rendus Biologies, Elsevier Masson, 2017, 340 (4), pp.226-237. 10.1016/j.crvi.2017.02.003. hal- 01681523 HAL Id: hal-01681523 https://hal.archives-ouvertes.fr/hal-01681523 Submitted on 23 Apr 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/315062117 Diversity of fungal assemblages in roots of Ericaceae in two Mediterranean contrasting ecosystems Article in Comptes rendus biologies · March 2017 DOI: 10.1016/j.crvi.2017.02.003 CITATIONS READS 0 37 7 authors, including: Ahmed Douaik Rachid Mrabet Institut National de Recherche Agronomique