Chemical Equilibrium and Reaction Modeling of Arsenic and Selenium in Soils
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Homework 5 Solutions
SCI 1410: materials science & solid state chemistry HOMEWORK 5. solutions Textbook Problems: Imperfections in Solids 1. Askeland 4-67. Why is most “gold” or “silver” jewelry made out of gold or silver alloyed with copper, i.e, what advantages does copper offer? We have two major considerations in jewelry alloying: strength and cost. Copper additions obviously lower the cost of gold or silver (Note that 14K gold is actually only about 50% gold). Copper and other alloy additions also strengthen pure gold and pure silver. In both gold and silver, copper will strengthen the material by either solid solution strengthening (substitutional Cu atoms in the Au or Ag crystal), or by formation of second phase in the microstructure. 2. Solid state solubility. Of the elements in the chart below, name those that would form each of the following relationships with copper (non-metals only have atomic radii listed): a. Substitutional solid solution with complete solubility o Ni and possibly Ag, Pd, and Pt b. Substitutional solid solution of incomplete solubility o Ag, Pd, and Pt (if you didn’t include these in part (a); Al, Co, Cr, Fe, Zn c. An interstitial solid solution o C, H, O (small atomic radii) Element Atomic Radius (nm) Crystal Structure Electronegativity Valence Cu 0.1278 FCC 1.9 +2 C 0.071 H 0.046 O 0.060 Ag 0.1445 FCC 1.9 +1 Al 0.1431 FCC 1.5 +3 Co 0.1253 HCP 1.8 +2 Cr 0.1249 BCC 1.6 +3 Fe 0.1241 BCC 1.8 +2 Ni 0.1246 FCC 1.8 +2 Pd 0.1376 FCC 2.2 +2 Pt 0.1387 FCC 2.2 +2 Zn 0.1332 HCP 1.6 +2 3. -

Chapter 15: Solutions
452-487_Ch15-866418 5/10/06 10:51 AM Page 452 CHAPTER 15 Solutions Chemistry 6.b, 6.c, 6.d, 6.e, 7.b I&E 1.a, 1.b, 1.c, 1.d, 1.j, 1.m What You’ll Learn ▲ You will describe and cate- gorize solutions. ▲ You will calculate concen- trations of solutions. ▲ You will analyze the colliga- tive properties of solutions. ▲ You will compare and con- trast heterogeneous mixtures. Why It’s Important The air you breathe, the fluids in your body, and some of the foods you ingest are solu- tions. Because solutions are so common, learning about their behavior is fundamental to understanding chemistry. Visit the Chemistry Web site at chemistrymc.com to find links about solutions. Though it isn’t apparent, there are at least three different solu- tions in this photo; the air, the lake in the foreground, and the steel used in the construction of the buildings are all solutions. 452 Chapter 15 452-487_Ch15-866418 5/10/06 10:52 AM Page 453 DISCOVERY LAB Solution Formation Chemistry 6.b, 7.b I&E 1.d he intermolecular forces among dissolving particles and the Tattractive forces between solute and solvent particles result in an overall energy change. Can this change be observed? Safety Precautions Dispose of solutions by flushing them down a drain with excess water. Procedure 1. Measure 10 g of ammonium chloride (NH4Cl) and place it in a Materials 100-mL beaker. balance 2. Add 30 mL of water to the NH4Cl, stirring with your stirring rod. -

Self-Assembly of a Colloidal Interstitial Solid Solution with Tunable Sublattice Doping: Supplementary Information
Self-assembly of a colloidal interstitial solid solution with tunable sublattice doping: Supplementary information L. Filion,∗ M. Hermes, R. Ni, E. C. M. Vermolen,† A. Kuijk, C. G. Christova,‡ J. C. P. Stiefelhagen, T. Vissers, A. van Blaaderen, and M. Dijkstra Soft Condensed Matter, Debye Institute for NanoMaterials Science, Utrecht University, Princetonplein 1, NL-3584 CC Utrecht, the Netherlands I. CRYSTAL STRUCTURE LS6 In the main paper we use full free-energy calculations to determine the phase diagram for binary mixtures of hard spheres with size ratio σS/σL = 0.3 where σS(L) is the diameter of the small (large) particles. The phases we considered included a structure with LS6 stoichiometry for which we could not identify an atomic analogue. Snapshots of this structure from various perspectives are shown in Fig. S1. FIG. S1: Unit cell of the binary LS6 superlattice structure described in this paper. Note that both species exhibit long-range crystalline order. The unit cell is based on a body-centered-cubic cell of the large particles in contrast to the face-centered-cubic cell associated with the interstitial solid solution covering most of the phase diagram (Fig. 1). II. PHASE DIAGRAM AT CONSTANT VOLUME 3 In the main paper, we present the xS − p representation of the phase diagram where p = βPσL is the reduced pressure, xS = NS/(NS + NL), NS(L) is the number of small (large) hard spheres, β = 1/kBT , kB is the Boltzmann constant, and T is the absolute temperature. This is the natural representation arising from common tangent construc- tions at constant pressure and the representation required for further simulation studies such as nucleation studies. -

Solid Solution Softening and Enhanced Ductility in Concentrated FCC Silver Solid Solution Alloys
UC Irvine UC Irvine Previously Published Works Title Solid solution softening and enhanced ductility in concentrated FCC silver solid solution alloys Permalink https://escholarship.org/uc/item/7d05p55k Authors Huo, Yongjun Wu, Jiaqi Lee, Chin C Publication Date 2018-06-27 DOI 10.1016/j.msea.2018.05.057 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Materials Science & Engineering A 729 (2018) 208–218 Contents lists available at ScienceDirect Materials Science & Engineering A journal homepage: www.elsevier.com/locate/msea Solid solution softening and enhanced ductility in concentrated FCC silver T solid solution alloys ⁎ Yongjun Huoa,b, , Jiaqi Wua,b, Chin C. Leea,b a Electrical Engineering and Computer Science University of California, Irvine, CA 92697-2660, United States b Materials and Manufacturing Technology University of California, Irvine, CA 92697-2660, United States ARTICLE INFO ABSTRACT Keywords: The major adoptions of silver-based bonding wires and silver-sintering methods in the electronic packaging Concentrated solid solutions industry have incited the fundamental material properties research on the silver-based alloys. Recently, an Solid solution softening abnormal phenomenon, namely, solid solution softening, was observed in stress vs. strain characterization of Ag- Twinning-induced plasticity In solid solution. In this paper, the mechanical properties of additional concentrated silver solid solution phases Localized homologous temperature with other solute elements, Al, Ga and Sn, have been experimentally determined, with their work hardening Advanced joining materials behaviors and the corresponding fractography further analyzed. Particularly, the concentrated Ag-Ga solid so- lution has been discovered to possess the best combination of mechanical properties, namely, lowest yield strength, highest ductility and highest strength, among the concentrated solid solutions of the current study. -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

CHEM 301 Assignment #2 Provide Solutions to the Following Questions in a Neat and Well-Organized Manner
CHEM 301 Assignment #2 Provide solutions to the following questions in a neat and well-organized manner. Reference data sources for any constants and state assumptions, if any. Due date: Thursday, Oct 20th, 2016 Attempt all questions. Only even numbers will be assessed. 1. A pond in an area affected by acid mine drainage is observed to have freshly precipitated Fe(OH)3(s) at pH 4. What is the minimum value of pe in this water? Use the appropriate pe-pH speciation diagram to predict the dominant chemical speciation of carbon, sulfur and copper under these conditions. 2. The solubility of FeS(s) in marine sediments is expected to be affected by the pH and the [H2S(aq)] in the surrounding pore water? Calculate the equilibrium concentration of Fe2+ (ppb) at pH 5 and pH 8, -3 assuming the H2S is 1.0 x 10 M. pKsp(FeS) = 16.84. + 2+ FeS(s) + 2 H (aq) ==== Fe (aq) + H2S 3. Boric acid is a triprotic acid (H3BO3); pKa1 = 9.24, pKa2 = 12.74 and pKa3 = 13.80. a) Derive an expression for the fractional abundance of H3BO3 as a function of pH b) Construct a fully labeled pH speciation diagram for boric acid over the pH range of 0 to 14 using Excel spreadsheet at a 0.2 pH unit interval. 4. At pCl- = 2.0, Cd2+ and CdCl+ are the only dominant cadmium chloride species with a 50% fractional abundance of each. + a) Calculate the equilibrium constant for the formation of CdCl (1). + - b) Derive an expression for the fractional abundance of CdCl as a function of [Cl ] and 1 – 4 for cadmium chloro species. -

Inorganic Arsenic Compounds Other Than Arsine Health and Safety Guide
OS INTERNATiONAL I'ROGRAMME ON CHEMICAL SAFETY Health and Safety Guide No. 70 INORGANIC ARSENIC COMPOUNDS OTHER THAN ARSINE HEALTH AND SAFETY GUIDE i - I 04 R. Q) UNEP UNITED NATIONS INTERNATIONAL ENVIRONMENT I'R( )GRAMME LABOUR ORGANISATION k\s' I V WORLD HEALTH ORGANIZATION WORLD HEALTH ORGANIZATION, GENEVA 1992 IPcs Other H EA LTH AND SAFETY GUIDES available: Aerytonitrile 41. Clii rdeon 2. Kekvau 42. Vatiadiuni 3 . I Bula not 43 Di meLhyI ftirmatnide 4 2-Buta101 44 1-Dryliniot 5. 2.4- Diehlorpheiioxv- 45 . Ac rylzi mule acetic Acid (2.4-D) 46. Barium 6. NIcihylene Chhride 47. Airaziiie 7 . ie,i-Buia nol 48. Benlm'.ie 8. Ep Ichioroli) Olin 49. Cap a 64 P. ls.ihutaiiol 50. Captaii I o. feiddin oeth N lene Si. Parai.tuat II. Tetradi ion 51 Diquat 12. Te nacelle 53. Alpha- and Betal-lexachloro- 13 Clils,i (lane cyclohexanes 14 1 kpia Idor 54. Liiidaiic IS. Propylene oxide 55. 1 .2-Diciilroetiiane Ethylene Oxide 5t. Hydrazine Eiulosiillaii 57. F-orivaldehydc IS. Die h lorvos 55. MLhyI Isobu I V I kcloiic IV. Pculaehloro1heiiol 59. fl-Flexaric 20. Diiiiethoaie 61), Endrin 2 1 . A iii in and Dick) 0in 6 I . I sh IIZiLI1 22. Cyperniellirin 62. Nicki. Nickel Caution I. and some 23. Quiiiloieiic Nickel Compounds 24. Alkthrins 03. Hexachlorocyclopeuladiene 25. Rsiiiethii ins 64. Aidicaib 26. Pyr rot ii,id inc Alkaloids 65. Fe nitrolhioit 27. Magnetic Fields hib. Triclilorlon 28. Phosphine 67. Acroleiii 29. Diiiiethyl Sull'ite 68. Polychlurinated hiphenyls (PCBs) and 30. Dc lianteth nil polyc h In ruiated letlilienyls (fs) 31. -

Chapter 18 Solutions and Their Behavior
Chapter 18 Solutions and Their Behavior 18.1 Properties of Solutions Lesson Objectives The student will: • define a solution. • describe the composition of solutions. • define the terms solute and solvent. • identify the solute and solvent in a solution. • describe the different types of solutions and give examples of each type. • define colloids and suspensions. • explain the differences among solutions, colloids, and suspensions. • list some common examples of colloids. Vocabulary • colloid • solute • solution • solvent • suspension • Tyndall effect Introduction In this chapter, we begin our study of solution chemistry. We all might think that we know what a solution is, listing a drink like tea or soda as an example of a solution. What you might not have realized, however, is that the air or alloys such as brass are all classified as solutions. Why are these classified as solutions? Why wouldn’t milk be classified as a true solution? To answer these questions, we have to learn some specific properties of solutions. Let’s begin with the definition of a solution and look at some of the different types of solutions. www.ck12.org 394 E-Book Page 402 Homogeneous Mixtures A solution is a homogeneous mixture of substances (the prefix “homo-” means “same”), meaning that the properties are the same throughout the solution. Take, for example, the vinegar that is used in cooking. Vinegar is approximately 5% acetic acid in water. This means that every teaspoon of vinegar contains 5% acetic acid and 95% water. When a solution is said to have uniform properties, the definition is referring to properties at the particle level. -

Code Chemical P026 1-(O-Chlorophenyl)Thiourea P081 1
Code Chemical P026 1-(o-Chlorophenyl)thiourea P081 1,2,3-Propanetriol, trinitrate (R) P042 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, (R)- P067 1,2-Propylenimine P185 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)- carbonyl]oxime 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a,-hexahydro-, P004 (1alpha,4alpha, 4abeta,5alpha,8alpha,8abeta)- 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a-hexahydro-, P060 (1alpha,4alpha, 4abeta,5beta,8beta,8abeta)- P002 1-Acetyl-2-thiourea P048 2,4-Dinitrophenol P051 2,7:3,6-Dimethanonaphth [2,3-b]oxirene, 3,4,5,6,9,9 -hexachloro-1a,2,2a,3,6,6a,7,7a- octahydro-, (1aalpha,2beta,2abeta,3alpha,6alpha,6abeta,7 beta, 7aalpha)-, & metabolites 2,7:3,6-Dimethanonaphth[2,3-b]oxirene, 3,4,5,6,9,9- hexachloro-1a,2,2a,3,6,6a,7,7a- P037 octahydro-, (1aalpha,2beta,2aalpha,3beta,6beta,6aalpha,7 beta, 7aalpha)- P045 2-Butanone, 3,3-dimethyl-1-(methylthio)-, O-[methylamino)carbonyl] oxime P034 2-Cyclohexyl-4,6-dinitrophenol 2H-1-Benzopyran-2-one, 4-hydroxy-3-(3-oxo-1- phenylbutyl)-, & salts, when present at P001 concentrations greater than 0.3% P069 2-Methyllactonitrile P017 2-Propanone, 1-bromo- P005 2-Propen-1-ol P003 2-Propenal P102 2-Propyn-1-ol P007 3(2H)-Isoxazolone, 5-(aminomethyl)- P027 3-Chloropropionitrile P047 4,6-Dinitro-o-cresol, & salts P059 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro- 3a,4,7,7a-tetrahydro- P008 4-Aminopyridine P008 4-Pyridinamine P007 5-(Aminomethyl)-3-isoxazolol 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- -

Chapter 4 Solution Theory
SMA5101 Thermodynamics of Materials Ceder 2001 Chapter 4 Solution Theory In the first chapters we dealt primarily with closed systems for which only heat and work is transferred between the system and the environment. In the this chapter, we study the thermodynamics of systems that can also exchange matter with other systems or with the environment, and in particular, systems with more than one component. First we focus on homogeneous systems called solutions. Next we consider heterogeneous systems with emphasis on the equilibrium between different multi-component phases. 4.1 WHAT IS A SOLUTION? A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level. A well-known example of a solution is salt water: The Na+, Cl- and H2O ions are intimately mixed at the atomic level. Many systems can be characterized as a dispersion of one phase within another phase. Although such systems typically contain more than one chemical component, they do not form a solution. Solutions are not limited to liquids: for example air, a mixture of predominantly N2 and O2, forms a vapor solution. Solid solutions such as the solid phase in the Si-Ge system are also common. Figure 4.1. schematically illustrates a binary solid solution and a binary liquid solution at the atomic level. Figure 4.1: (a) The (111) plane of the fcc lattice showing a cut of a binary A-B solid solution whereby A atoms (empty circles) are uniformly mixed with B atoms (filled circles) on the atomic level. -

Solid-Liquid Partition Coefficients, Rcjs: What's the Value and When Does It Matter?
Radioprotection - Colloques, volume 37, Cl (2002) Cl-225 Solid-liquid partition coefficients, rCjs: What's the value and when does it matter? M.I. Sheppard and S.C. Sheppard ECOMatters, 24 Aberdeen Avenue, P.O. Box 430, Pinawa, Manitoba ROE 1L0, Canada Abstract. Environmental risk assessments binge on our ability to predict the fete and mobility of radionuclides and metals in terrestrial soils and aquatic sediments. Solid-solution partitioning (the Kd approach), despite its shortcomings, has been used extensively. Much attention has been devoted to grooming the existing key compendia for values applicable for each nuclear risk assessment carried out worldwide. This appears to be an important task. For example, soil Kd values for a single nuclide can vary over several orders of magnitude, yet the soil Kd value is the most important parameter in the soil leaching model. Similarly, plant uptake depends primarily on the nuclide present in solution phase. Despite this apparent sensitivity, our experience has shown that risk assessors dwell too much on the precision of the Kd value for all nuclides. This paper discusses the effect of the Kd value on the resulting soil concentration during leaching and identifies those radionuclides and assessment conditions where a precise value is required. Only those radionuclides that typically have a soil Kd value of 10 LAg or less (Tc, CL I, As) need be accurately described for timeframes of up to 10,000 years for desired soil model prediction outcomes (soil concentrations within two-fold). 1. INTRODUCTION Environmental risk assessments of radionuclides and metals are key to acceptance and sustainability of industrial progress and the disposal of industry's wastes, particularly in the nuclear and imriing and metal smelting industry. -
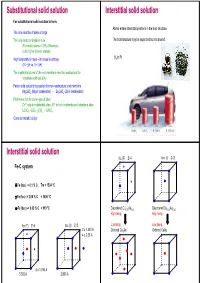
Substitutional Solid Solution Interstitial Solid Solution for Substitutional Solid Solution to Form: Atoms Enters Intersitital Positions in the Host Structure
Substitutional solid solution Interstitial solid solution For substitutional solid solution to form: Atoms enters intersitital positions in the host structure. The ions must be of same charge The ions must be similar in size. The host structure may be expanded but not altered. (For metal atoms < 15% difference) (a bit higher for non-metals) High temperature helps – increase in entropy H2 in Pt (0 > ∆H vs. 0 < ∆H) The crystal structures of the end members must be isostructural for complete solid solubility Partial solid solubility is possible for non-isostructural end members Mg2SiO4 (Mg in octahedras) - Zn2SiO4 (Zn in tetrahedras) Preference for the same type of sites Cr3+ only in octahedral sites, Al3+ in both octahedra and tetraheda sites LiCrO2 -LiCr1-xAlxO2 - LiAlO2 Consider metallic alloys Mg2NH4 LaNi5H6 H2 (liquid) H2 (200 bar) Interstitial solid solution fcc (F) Z=4 bcc (I)Z=2 Fe-C system δ-Fe (bcc) -> 0.1 % C Tm = 1534 °C γ-Fe (fcc) -> 2.06 % C < 1400 °C α-Fe (bcc) -> 0.02 % C < 910 °C Disordered Cu0.75Au0.25 Disordered Cu0.50Au0.50 High temp High temp fcc (F) Z=4 bcc (I)Z=2 Low temp Low temp 2 x 1.433 Å Ordered Cu3Au Ordered CuAu 4 x 2.03 Å 6 x 1.796 Å 3.592 Å 2.866 Å Aliovalent substitution Aliovalent substitution Substitution with ions of different charge 1 Cation vacancies, Substitution by higher valence Need charge compensation mechanism Preserve charge neutrality by leaving out more cations than those that are replaced. Substitution by higher valence cations NaCl dissolves CaCl2 by: Na1-2xCaxVxCl 1 2 Ca2+ vil have a net excess charge of +1 in the structure and attract Na+ vacancies which have net charge -1 Cation vacancies Interstitial anions 2+ 3+ Mg may be replaced by Al : Mg1-3xAl2+2xVxO4 Substitution by lower valence cations 3 4 Anion vacancies Interstitial cations Aliovalent substitution Aliovalent substitution 2 Interstitial anions, Substitution by higher valence 3 Anion vacancies, Substitution by lower valence Preserve charge neutrality by inserting more anions interstitially.