Harvesting the Radial Artery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Morphology of Common Interosseous Artery and Its Clinical
Scholars Journal of Applied Medical Sciences (SJAMS) ISSN 2320-6691 (Online) Sch. J. App. Med. Sci., 2015; 3(3B):1126-1131 ISSN 2347-954X (Print) ©Scholars Academic and Scientific Publisher (An International Publisher for Academic and Scientific Resources) www.saspublisher.com Research Article The Morphology of Common Interosseous Artery and its Clinical Significance Waseem Al Talalwah1*, Dereje Getachew2 and Roger Soames3 1King Abdullah International Medical Research Center / King Saud bin Abdulaziz University for Health Sciences, College of Medicine, Department of Basic Medical Sciences Hospital – NGHA, Riyadh, P.O. Box 3660, Riyadh 2Anatomy Department, College of Medicine and Health sciences, Hawassa University, Awassa, 1560 3Centre for Anatomy and Human Identification, College of Art, Science and Engineering, University of Dundee Dundee, DD1 5EH, UK *Corresponding author Dr. Waseem Al-Talalwah Abstract: The common interosseous artery is main branch the ulnar artery which divides into anterior and posterior interosseous branches. The current study investigates common interosseous artery and its branch to provide detailed information regarding the morphology which would be of use to clinicians, orthopaedic surgeons, plastic surgeons and anatomists. Routine dissections of the right and left upper limb of 34 adult cadavers (20 male and 14 female: mean age 78.9 year) were undertaken. The common interosseous artery presents in 67.6% whereas it is congenital absence in 32.4%. The origin distance of bifurcation of common interosseous from the ulnar artery origin is between 33.11 and 33.45 mm. The anterior and posterior interosseous arteries present in 98.5% and 92.9% whereas they are congenital absence in 1.5% and 7.1% respectively in total cases. -

Normal Blood Supply to Equine Radii and Its Response to Various Cerclage Devices
NORMAL BLOOD SUPPLY TO EQUINE RADII AND ITS RESPONSE TO VARIOUS CERCLAGE DEVICES by KAREN ANN NYROP B.S., Montana State University, 1977 D.V.M., University of Minnesota, 1981 A MASTER'S THESIS Submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Surgery and Medicine Kansas State University Manhattan, Kansas 1934 Approved by: H. Rodney Ferguson, jy.V.M, Ph.D. Major Professor /_£ A11EDE Tt.0443 yif TABLE OF CONTENTS Page //17 LIST OF FIGURES Hi ACKNOWLEDGEMENTS i* INTRODUCTION v LITERATURE REVIEW 1 Blood Supply of a Long Bone 1 Microangiography and Related Infusion Techniques 6 Cerclage Devices 9 2 MATERIALS AND METHODS 1 * RESULTS 18 Clinical Observations 18 Radiographic Evaluations 18 Gross Postmortem Evaluations 18 Microangiographic Evaluations 19 DISCUSSION 21 SUMMARY AND CONCLUSIONS 26 FOOTNOTES 28 BIBLIOGRAPHY 29 APPENDIX 32 ii . LIST OF FIGURES Page 1 Circumferential Partial Contact Band 32 2. Circumferential Partial Contact Band , sideview 34 3. Crossection of radius with Circumferential Partial Contact band 36 4. Anterior - posterior Radiograph of the radius of Pony 1 , Group I 38 5. Lateral - medial radiograph of the radius of Pony 1 , Group I 40 6. Microangiograph, Longitudinal section, of Control Pony 3 , Group I 42 7. Microangiograph, Crossectional view, of Control Pony 3 , Group I 44 8. Microangiograph, Crossectional view through a Cerclage Wire of Pony 1 , Group I 46 9. Microangiograph, Crossectional view through a Parham-Martin band of Pony 1 , Group I 48 10. Microangiograph, Longitudinal view with three cerclage devices in position in Pony 2, Group I 50 11. -

01 Natsis.P65
Folia Morphol. Vol. 68, No. 4, pp. 193–200 Copyright © 2009 Via Medica R E V I E W A R T I C L E ISSN 0015–5659 www.fm.viamedica.pl Persistent median artery in the carpal tunnel: anatomy, embryology, clinical significance, and review of the literature K. Natsis1, G. Iordache2, I. Gigis1, A. Kyriazidou1, N. Lazaridis1, G. Noussios3, G. Paraskevas1 1Department of Anatomy, Medical School, Aristotle University of Thessaloniki, Greece 2University of Medicine and Pharmacy of Craiova, Romania 3Laboratory of Anatomy, Department of Physical Education and Sport Sciences (Serres), Aristotle University of Thessaloniki, Greece [Received 5 June 2009; Accepted 16 September 2009] The median artery usually regresses after the eighth week of intrauterine life, but in some cases it persists into adulthood. The persistent median artery (PMA) pas- ses through the carpal tunnel of the wrist, accompanying the median nerve. During anatomical dissection in our department, we found two unilateral cases of PMA originating from the ulnar artery. In both cases the PMA passed through the carpal tunnel, reached the palm, and anastomosed with the ulnar artery, forming a medio-ulnar type of superficial palmar arch. In addition, in both cases we observed a high division of the median nerve before entering the carpal tunnel. Such an artery may result in several complications such as carpal tunnel syndrome, pronator syndrome, or compression of the anterior interosseous nerve. Therefore, the presence of a PMA should be taken into consideration in clinical practice. This study presents two cases of PMA along with an embryological explanation, analysis of its clinical significance, and a review of the literature. -

Anatomical Basis and Clinical Application of the Ulnar Forearm Free Flap for Head and Neck Reconstruction
The Laryngoscope VC 2012 The American Laryngological, Rhinological and Otological Society, Inc. Anatomical Basis and Clinical Application of the Ulnar Forearm Free Flap for Head and Neck Reconstruction Jung-Ju Huang, MD; Chih-Wei Wu, MD; Wee Leon Lam, MB ChB, MPhil, FRCS (Plast); Dung H. Nguyen, MD; Huang-Kai Kao, MD; Chia-Yu Lin, MSc; Ming-Huei Cheng, MD, MBA Objectives/Hypothesis: This study was designed to investigate the anatomical features and applications of the ulnar forearm flap in head and neck reconstructive surgery. Study Design: A prospective study was designed to include 50 ulnar forearm free flap transplants in 50 patients. Patient defects requiring reconstructive surgery involved the buccal mucosa, tongue, floor of the mouth, upper or lower gums, lips, soft palate, and scalp. Twenty ulnar forearm flaps were analyzed along the entire ulnar artery to determine the anatomy and distribution of the ulnar artery septocutaneous perforators. Results: All 50 flaps were successfully transplanted into their respective sites. The mean diameters of the ulnar artery and vein were 2.3 6 0.6 mm and 1.7 6 0.6 mm, respectively. Arterial and venous size mismatch was experienced in 12 and 33 flaps, respectively. The mean number of sizable perforators was 4.3 6 1.2, and most of the first perforators were located within 5 cm of the proximal wrist crease. None of the patients experienced long-term complications concerning the ulnar nerve. Conclusions: The ulnar forearm flap is a reliably consistent source of free flap transfer because it harbors constant sep- tocutaneous perforators and produces minimal donor site morbidities for head and neck reconstructive surgery. -

Volume-8, Issue-3 July-Sept-2018 Coden:IJPAJX-CAS-USA
Volume-8, Issue-3 July-Sept-2018 Coden:IJPAJX-CAS-USA, Copyrights@2018 ISSN-2231-4490 Received: 8th June-2018 Revised: 15th July-2018 Accepted: 16th July-2018 DOI: 10.21276/Ijpaes http://dx.doi.org/10.21276/ijpaes Case Report VARIANT ARTERIAL PATTERN IN THE FOREARM WITH ITS EMBRYOLOGICAL BASIS Vaishnavi Joshi and Dr. Shaheen Sajid Rizvi Department of Anatomy, K. J. Somaiya Medical College, Somaiya, Ayurvihar, Eastern Express Highway, Sion, Mumbai-400 022 ABSTRACT: During routine dissection for the first MBBS students, we observed that the radial artery was absent in the right upper limb of a 70 years old, donated embalmed male cadaver in the Department of Anatomy, K.J.Somaiya Medical College, Sion. In the lower part of the arm, brachial artery divided into ulnar and common Interosseous artery. Anterior interosseous artery was large in size. Deep to pronator quadratus, it turned laterally and reached the dorsum of the hand, where its lateral branch supplied the thumb and index finger and its medial branch dipped into the palm at the second inter-metacarpal space. Superficial palmar arch was absent. Digital arteries from the medial and lateral branches of ulnar artery supplied the fingers. Embryological basis is presented. Key words: Brachial artery, Anterior interosseous artery, Common Interosseous artery, Radial artery, ulnar artery *Corresponding autor: Dr. Shaheen Sajid Rizvi, Department of Anatomy, K. J. Somaiya Medical College, Somaiya, Ayurvihar, Eastern Express Highway, Sion, Mumbai-400 022; Email : rizvishaheen68@ gmail.com Copyright: ©2018 Dr. Shaheen Sajid Rizvi. This is an open-access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited INTRODUCTION The main artery of the arm, the brachial artery divides at the level of the neck of the radius into radial and ulnar arteries. -

Branches of Ulnar Artery in Human Fetuses: Anatomical and Morphometric Study
Surgical and Radiologic Anatomy (2019) 41:1325–1332 https://doi.org/10.1007/s00276-019-02297-6 ORIGINAL ARTICLE Branches of ulnar artery in human fetuses: anatomical and morphometric study Selda Yildiz1 · Necdet Kocabiyik1 · Ozlem Elvan2 · Bulent Yalcin1 · Ayhan Comert3 Received: 9 May 2018 / Accepted: 26 July 2019 / Published online: 17 September 2019 © Springer-Verlag France SAS, part of Springer Nature 2019 Abstract Purpose This study was conducted to demonstrate morphological pattern of the ulnar artery and to evaluate morphometri- cally its anatomical branching pattern in human fetuses. Methods Branching pattern of ulnar artery was evaluated on 121 upper limbs of dissected 63 of formalin-fxed fetus cadav- ers with gestational age ranging from 17 to 40 weeks. In order to obtain second and third trimester data, according to their gestational age, two groups were determined. Results In 79 of all 121 upper limbs (65%) ulnar artery gave anterior and posterior ulnar recurrent arteries as separate branches. In this study frequency of presence of a median artery was 46.28% among total examined 121 upper limbs. Median arteries originated from ulnar artery (3.57%) and from the common interosseous artery (53.57%) and anterior interosseous artery (42.85%). Mean distances of the measured parameters were demonstrated according to the gestational age and dif- ferences between group I (second trimester) and group II (third trimester). No statistical diference for groups was observed for gender and between right and left sides. Conclusions Ulnar artery shows predictable patterns during second and third trimester of fetal period and can be suitable access efective alternative for diagnostic and therapeutic coronary interventions. -

Arteries of The
This document was created by Alex Yartsev ([email protected]); if I have used your data or images and forgot to reference you, please email me. Arteries of the Arm st The AXILLARY ARTERY begins at the border of the 1 rib as a continuation of the subclavian artery Subclavian artery The FIRST PART stretches between the 1st rib and the medial border of pectoralis minor. First rib It has only one branch – the superior thoracic artery Superior thoracic artery The SECOND PART lies under the pectoralis Thoracoacromial artery minor; it has 2 branches: Which pierces the - The Thoracoacromial artery costocoracoid membrane - The Lateral Thoracic artery deep to the clavicular head The THIRD PART stretches from the lateral border of pectoralis major of pectoralis minor to the inferior border of Teres Major; it has 3 branches: Pectoralis major - The Anterior circumflex humeral artery - The Posteror circumflex humeral artery Pectoralis minor - The Subscapular artery Axillary nerve Posterior circumflex humeral artery Lateral Thoracic artery Travels through the quadrangular space together Which follows the lateral with the axillary nerve. It’s the larger of the two. border of pectoralis minor onto the chest wall Anterior circumflex humeral artery Passes laterally deep to the coracobrachialis and Circumflex scapular artery the biceps brachii Teres Major Passes dorsally between subscapularis and teres major to supply the dorsum of the scapula Profunda Brachii- deep artery of the arm Thoracodorsal artery Passes through the lateral triangular space (with Goes to the inferior angle of the scapula, the radial nerve) into the posterior compartment Triceps brachii supplies mainly the latissimus dorsi of the arm. -

Arterial Damages in Acute Elbow Dislocations: Which Diagnostic Tests Are Required? Christoph Lutter,1,2 Ronny Pfefferkorn,2 Volker Schoeffl2
Rare disease BMJ Case Reports: first published as 10.1136/bcr-2016-216336 on 19 July 2016. Downloaded from CASE REPORT Arterial damages in acute elbow dislocations: which diagnostic tests are required? Christoph Lutter,1,2 Ronny Pfefferkorn,2 Volker Schoeffl2 1CVPath Institute, SUMMARY radiographs. After reduction, the left arm was Gaithersburg, Maryland, USA Blunt vessel injuries of peripheral arteries caused by a immobilised in a splint and the patient was dis- 2Department of Sports Orthopedics, Sports Medicine, direct trauma are rare. Studies have described the charged. Several days later, MRI was performed, Sports Traumatology, frequency of arterial ruptures following closed elbow showing a partial detachment of the extensor Department for Orthopedics dislocations in 0.3–1.7% of all cases. However, arterial muscles from the radial epicondyle and a medial and Traumatology, Klinikum damage does not always necessarily appear as a sided ligamental lesion (figure 1). In addition, a Bamberg, Bamberg, Germany complete rupture of the vessel with a loss of peripheral partial rupture of the brachial muscle tendon and a Correspondence to circulation and ischaemic symptoms; a relatively strong haematoma within the brachial muscle were Dr Christoph Lutter, periarticular system of collaterals can maintain detected. christoph.lutter@googlemail. circulation. Furthermore, the traumatic dislocation can com also cause intimal tears, arterial dissections and INVESTIGATIONS Accepted 5 July 2016 aneurysms or thrombosis. In all cases of vessel injury, including total disruption, a peripheral pulse might still Two weeks later, the patient was seen in our out- be palpable. 3 weeks after an acute elbow dislocation, patient clinic. The left arm was still immobilised in we have diagnosed a patient with a long-segment a 110° extension position in the elbow, and an old stenosis of the brachial artery and a thrombosis of the haematoma was visible on the medial side of the radial artery. -

A Series of Study of Anatomic Variation on Arterial System
Article ID: WMC003513 ISSN 2046-1690 A Series Of Study Of Anatomic Variation On Arterial System Corresponding Author: Dr. Prakash Baral, Associate Professor, B.P.Koirala Institute Of Health Sciences,Department of Human Anatomy - Nepal Submitting Author: Dr. Sarun Koirala, Assistant Professor, Department of Human Anatomy, BP Koirala Institute of Health Sciences, 56700 - Nepal Article ID: WMC003513 Article Type: Original Articles Submitted on:24-Jun-2012, 12:40:17 PM GMT Published on: 26-Jun-2012, 09:14:28 PM GMT Article URL: http://www.webmedcentral.com/article_view/3513 Subject Categories:ANATOMY Keywords:Upper limb, Arterial variation, Axillary, Forearm, Palmar level. How to cite the article:Baral P, Koirala S. A Series Of Study Of Anatomic Variation On Arterial System. WebmedCentral ANATOMY 2012;3(6):WMC003513 Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License(CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Source(s) of Funding: None Competing Interests: Nil Additional Files: A Series Of Study Of Anatomic Variation On A Series Of Study Of Anatomic Variation On WebmedCentral > Original Articles Page 1 of 7 WMC003513 Downloaded from http://www.webmedcentral.com on 16-Feb-2016, 01:37:38 PM A Series Of Study Of Anatomic Variation On Arterial System Author(s): Baral P, Koirala S Abstract palmar branch of radial artery whereas the radial artery forms the deep palmar arch with the deep branch of ulnar artery.2 Many authors have published different series of reports about arterial anomalies of The arteries supplying the upperlimb exhibit lots of the upper extremities. -

A Variant Branch of the Axillary Artery Impacting in the Superficial Palmar Arch Composition
Case Report Annals of Clinical Anatomy Published: 25 Jun, 2018 A Variant Branch of the Axillary Artery Impacting in the Superficial Palmar Arch Composition Expedito S Nascimento Jr*, Jorge Landivar Coutinho, Karolina Duarte Rego, Jeovana Pinheiro F Souza, Marina Maria VF Caldas, Naryllenne Maciel Araújo, Wylqui Mikael G Andrade and Fernando Vagner Lobo Ladd Department of Morphology, Bioscience Center, Federal University of Rio Grande do Norte, Brazil Abstract During routine dissection of an approximately 60-year-old female cadaver for the undergraduate medical students at Morphology Department of Federal University of Rio Grande do Norte, Brazil, was observed a variant branch originated from the second part of the axillary artery. The second part of the right axillary artery gave rise to aberrant brachial artery that travels down superficially in the medial aspect of the upper limb. Furthermore, this superficial brachial artery terminates in the superficial palmar arch completely replacing the ulnar artery at this level. Variations in the upper limb arterial distribution are notably important for surgeons performing interventional or diagnostic in vascular diseases. Keywords: Axillary artery; Superficial palmar arch; Anatomic variation Introduction The axillary artery is a continuation of the subclavian artery, extending from the outer border of the first rib to the lower border of the teres major muscle where it continuous as brachial artery. Using as a reference the pectoralis minor muscle, the axillary artery could be divided into three parts: the first part extends from the outer board of the first rib to the superior board of the pectoralis OPEN ACCESS minor muscle; the second part is entirely covered by the pectoralis minor muscle; and the third part extends from the inferior border of the pectoralis minor muscle to the lower board of the teres *Correspondence: major muscle [1]. -
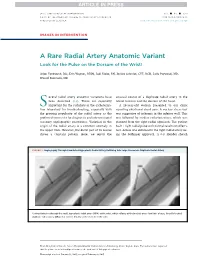
A Rare Radial Artery Anatomic Variant Look for the Pulse on the Dorsum of the Wrist!
JACC: CARDIOVASCULAR INTERVENTIONS VOL. - ,NO.- ,2017 ª 2017 BY THE AMERICAN COLLEGE OF CARDIOLOGY FOUNDATION ISSN 1936-8798/$36.00 PUBLISHED BY ELSEVIER http://dx.doi.org/10.1016/j.jcin.2017.02.026 IMAGES IN INTERVENTION A Rare Radial Artery Anatomic Variant Look for the Pulse on the Dorsum of the Wrist! Arjun Venkatesh, BSC, Erin Wagner, RT(R), Jodi Klebe, RN, Jessica Schmidt, CVT, RCIS, Laila Payvandi, MD, Wassef Karrowni, MD everal radial artery anatomic variations have unusual course of a duplicate radial artery in the S been described (1,2). These are especially lateral forearm and the dorsum of the hand. important for the radialists in the catheteriza- A56-year-oldwomanpresentedtoourclinic tion laboratory for troubleshooting, especially with reporting exertional chest pain. A nuclear stress test the growing popularity of the radial artery as the was suggestive of ischemia in the inferior wall. This preferred access site for diagnostic and interventional was followed by cardiac catheterization, which was coronary angiographic procedures. Variation in the planned from the right radial approach. The patient origin of the radial artery is a common anomaly in had 1þ right radial pulse with normal results on Allen’s the upper limb. However, the distal part of its course test. Access was obtained in the right radial artery us- shows a constant pattern. Here, we report the ing the Seldinger approach. A 6-F Slender sheath FIGURE 1 Angiography Through CannulatedHypoplasticRadialArteryRefluxing Into Large Anomalous Duplicate Radial Artery Contrast injection through the small, hypoplastic radial artery (HRA) at the wrist, which refluxes at the brachial artery level into a large, laterally located duplicate radial artery (DRA) that crosses distally to the dorsum of the wrist. -

Families Who Code Together Stay Together 50 Test Yourself Members Confess That Their Passion for Coding Doesn’T Stay at the Office
June 2008 (clockwise) Clifton L. Jones, CPC, CCP Joyce L. Jones CPC, CPC-H, CCS-P, CPC-ASC, CNT Traci Linn, CPC Families Who Beth Wolf, BA, CPC Code Together Stay Together Plus: Work of a Coder Survey • MPFS • Medical Policy • RACs • Advanced Beneficiary Notices CodingWebU.comCodingWebU.com TheThe NewNew HomeHome ofof thethe AnnualAnnual CEUCEU CodingCoding ScenariosScenarios && thethe “Nuts“Nuts andand Bolts”Bolts” ofof E&ME&M CodingCoding SeriesSeries CPC® Part 1 $85 for 9 CEUs CPC® Part 2 $85 for 9 CEUs CPC-H® $75 for 7 CEUs CPC-P® $85 for 8 CEUs E&M $65 for 6 CEUs Orthopedics $75 for 7 CEUs 2008 and 2007 Versions are Currently Available Local Chapter Gifts CodingWebU.comCodingWebU.com willwill givegive eacheach chapterchapter giftgift certificatescertificates toto bebe usedused asas rafflesraffles oror givegive awayaway atat youryour locallocal oror regionalregional conferences.conferences. ContactContact CodingWebU.comCodingWebU.com forfor moremore details.details. CodingWebU.com™ Providing Quality Education at Affordable Prices (484) 433-0495 www.CodingWebU.com contents 9 33 47 June 2008 In Every Issue [contents] 5 Letter From the Vice President 7 Letter From the NAB President 12 Letters to the Editor 15 Coding News 23 Kudos 24 42 Extreme Coding Features 8 ABN: Shift Responsibility to Patients the Correct Way Belinda S. Frisch, CPC, gives you information on Advanced Beneficiary Notice of Non-coverage (ABN) to correctly notify beneficiaries that Medicare is not likely to Education cover specific services. 16 Top 10 Reasons for Employers 18 Stay Current with April 2008 Medicare Physician Fee Schedule Updates to Participate in Project Xtern MPFS changes quarterly, tugged in various directions by several initiatives.