Are Competitive Effects of Native Species on an Invader Mediated by Water Availability?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
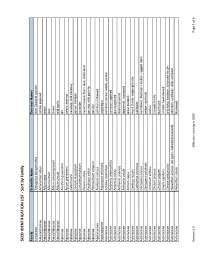
SEED IDENTIFICATION LIST - Sort by Family
SEED IDENTIFICATION LIST - Sort by Family Family Scientific Name Common Names Aizoaceae Tetragonia tetragonoides New Zealand spinach Amaranthaceae Amaranthus albus tumble pigweed Amaryllidaceae Allium cepa onion Amaryllidaceae Allium porrum leek Amaryllidaceae Allium schoenoprasum chives Amaryllidaceae Allium vineale wild garlic Apiaceae Anethum graveolens dill Apiaceae Apium graveolens celery, celeriac Apiaceae Carum carvi caraway; wild caraway Apiaceae Conium maculatum poison hemlock Apiaceae Coriandrum sativum coriander Apiaceae Daucus carota carrot; Queen Ane's lace; wild carrot Apiaceae Pastinaca sativa parsnip; wild parsnip Apiaceae Petroselinum crispum parsley Apocynaceae Asclepias syriaca common milkweed Asparagaceae Asparagus officinalis asparagus Asteraceae Achillea millefolium common yarrow, woolly yarrow Asteraceae Ambrosia artemisiifolia common ragweed Asteraceae Ambrosia trifida giant ragweed Asteraceae Anthemis arvensis field chamomile Asteraceae Anthemis cotula dogfennel, mayweed Asteraceae Arctium lappa great burdock Asteraceae Carduus nutans musk thistle, nodding thistle Asteraceae Carthamus tinctorius safflower Asteraceae Centaurea cyanus cornflower, bachelor's button, ragged robin Asteraceae Centaurea solstitialis yellow starthistle Asteraceae Cichorium endivia endive Asteraceae Cirsium arvense Canada thistle Asteraceae Cirsium vulgare bull thistle Asteraceae Crepis capillaris smooth hawksbeard Asteraceae Cynara cardunculus artichoke, cardoon, artichoke thistle Asteraceae Helianthus annuus (all types, cultivated and -

WRA Species Report
Family: Aizoaceae Taxon: Tetragonia tetragonoides Synonym: Demidovia tetragonoides Pall. (basionym) Common Name New Zealand spinach Tetragonia expansa Murray Questionaire : current 20090513 Assessor: Chuck Chimera Designation: H(Hawai'i) Status: Assessor Approved Data Entry Person: Chuck Chimera WRA Score 7 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- High substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 y 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 y 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 y 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see y Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see Appendix 2) 401 Produces spines, thorns or burrs y=1, n=0 n 402 Allelopathic y=1, n=0 n 403 Parasitic y=1, n=0 n 404 Unpalatable to grazing animals y=1, n=-1 n 405 Toxic -

Asian Vegetables & Herbs Easy to Grow in Southern California
Florence Nishida lagreengrounds.org March 2020 Asian Vegetables & Herbs Easy to Grow in Southern California Name Planting Need Pot Comments time support ok Legumes Snow Peas (Pisum sativum, var. macrocarpon Cool y/n y Bush and pole varieties Spr/Fall Pea shoots – various Spr/Fall y y Pick young, not tendrils Soya Bean (Glycine max) Spr n n Pull up plant for dried Yard Long Bean (Vigna unguiculata ssp. Sesquipedalis) Warm y y Long harvest, keep picked spr Lab Lab Beans (Lab Lab purpureus, Dolichos lab lab), Warm y/n n Can be a perennial if no Hyacinth Bean spr, frost. Contains cyanogenic summer glycosides, eat young or boil mature beans. Winged Bean/Pea (Tetragonolobus purpureus) Cool n y Fast maturing, pretty Brassicas, Headed Chinese cabbage, Napa cabbage (Brassica rapa var. Late n n Fresh – mild sweet flavor, pekinensis) summer, sev. cultivars fall Pak choi/ Bok choy (Brassica rapa var. chinensis), Spring n y Fast-grower, good for celery cabbage inter-cropping, harvest whole or lvs Rosette pak choi (Brassica chinensis var. narinosa), Mid n y Neat, compact, harvest ‘Tatsoi’, ‘Taisai’ summer, leaves or whole fall Flowering Stalk Brassicas Chinese broccoli (Brassica oleracea var. alboglabra) Late spr- n n Young flowering stems and ‘Gai lan’ fall buds Komatsuna (Brassica rapa var. komatsuna) Mustard Late n n Flavor bet. cabbage and spinach spr-fall mustard; eat whole or leaves Mustard Greens Mizuna (Brassica rapa var. japonica), green, red Fall- n y Mild flavored, pretty, pick varieties spring leaves Japanese Giant Red Mustard (Brassica juncea), Osaka Fall-Sprin n y/n Gorgeous, very spicy-hot Purple Mustard (B. -

Tetragonia Tetragonoides (Pall.) Kuntze (New Zealand Spinach) Prevents Obesity and Hyperuricemia in High-Fat Diet-Induced Obese Mice
nutrients Article Tetragonia tetragonoides (Pall.) Kuntze (New Zealand Spinach) Prevents Obesity and Hyperuricemia in High-Fat Diet-Induced Obese Mice Young-Sil Lee 1, Seung-Hyung Kim 2 ID , Heung Joo Yuk 1, Geung-Joo Lee 3,* and Dong-Seon Kim 1,* 1 Herbal Medicine Research Division, Korea Institute of Oriental Medicine, 1672 Yuseong-daero, Yuseong-gu, Dajeon 34054, Korea; [email protected] (Y.-S.L.); [email protected] (H.J.Y.) 2 Institute of Traditional Medicine and Bioscience, Daejeon University, 62 Daehak-ro, Dong-gu, Daejeon 34520, Korea; [email protected] 3 Department of Horticulture, Chungnam National University, 99 Daehak-ro, Yuseong-gu, Daejeon 34134, Korea * Correspondence: [email protected] (G.-J.L.); [email protected] (D.-S.K.); Tel.: +82-42-821-5734 (G.-J.L.); +82-42-868-9639 (D.-S.K.) Received: 6 July 2018; Accepted: 10 August 2018; Published: 14 August 2018 Abstract: Tetragonia tetragonoides (Pall.) Kuntze, called New Zealand spinach (NZS), is an edible plant used in salad in Western countries and has been used to treat gastrointestinal diseases in traditional medicine. We examined the anti-obesity and anti-hyperuricemic effects of NZS and the underlying mechanisms in high-fat diet (HFD)-induced obese mice. Mice were fed a normal-fat diet (NFD); high-fat diet (HFD); HFD with 75, 150, or 300 mg/kg NZS extract; or 245 mg/kg Garcinia cambogia (GC) extract. NZS decreased body weight gain, total white adipose tissue (WAT), liver weight, and size of adipocytes and improved hepatic and plasma lipid profiles. With NZS, the plasma levels of the leptin and uric acid were significantly decreased while the levels of the adiponectin were increased. -

From Tetragonia Tetragonoides in Lps-Induced Raw 264.7 Cells
EXCLI Journal 2017;16:521-530 – ISSN 1611-2156 Received: January 05, 2017, accepted: March 27, 2017, published: April 18, 2017 Original article: ANTI-INFLAMMATORY ACTIVITY OF HYDROSOLS FROM TETRAGONIA TETRAGONOIDES IN LPS-INDUCED RAW 264.7 CELLS Eun-Yi Koa,1, Su-Hyeon Choa,b,1, Kyungpil Kangc, Gibeom Kimc, Ji-Hyeok Leeb, You-Jin Jeonb, Daekyung Kima, Ginnae Ahnd, Kil-Nam Kime* a Jeju Center, Korea Basic Science Institute (KBSI), Jeju 690-140, Republic of Korea b Department of Marine Life Science, Jeju National University, Jeju 690-756, Republic of Korea c BKSU Lnc. Jeju , 63243, Republic of Korea d Department of Marine Bio-food science, College of Fisheries and Ocean Sciences, Chonnam National University, 500-749, Republic of Korea e Chuncheon Center, Korea Basic Science Institute (KBSI), Chuncheon 24341, Republic of Korea 1 These authors contributed equally to this work. * corresponding author: Tel.: +82-33-815-4607; Fax: +82-33-255-7273; E-mail: [email protected] (KN Kim) http://dx.doi.org/10.17179/excli2017-121 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). ABSTRACT The present study was performed to investigate the anti-inflammatory activity of Tetragonia tetragonoides hydro- sols (TTH) and its underlying mechanism in lipopolysaccharide (LPS)-induced RAW 264.7 cells. Gas chromatog- raphy (GC) coupled with mass spectrometry and retention index calculations showed that TTH were mainly com- posed of tetratetracontane (29.5 %), nonacosane (27.6 %), and oleamide (17.1 %). TTH significantly decreased the production of nitric oxide (NO), prostaglandin E2 (PGE2), interleukin (IL)-6, and IL-1β in LPS-stimulated RAW 264.7 cells. -

SPECIES REPORT for ASHY STORM-PETREL (Oceanodroma Homochroa)
1 SPECIES REPORT FOR ASHY STORM-PETREL (Oceanodroma homochroa) PHOTO CREDIT: ILANA NIMZ U.S. Fish and Wildlife Service (Service) Bay-Delta Fish and Wildlife Office, Sacramento, California 9/16/13 2 EXECUTIVE SUMMARY The ashy storm-petrel (Oceanodroma homochroa) is a small seabird whose known at-sea distribution ranges from about the California-Oregon Border to Islas San Benitos, Mexico. The 32 known breeding sites of the ashy storm-petrel stretch from Point Cabrillo, Mendocino County, California to Islas Todos Santos Island, Ensenada, Mexico. More than 90 percent of the population breeds in two population centers at Southeast (SE) Farallon Island and in the California Channel Islands. Anacapa, San Miguel, Santa Cruz, Santa Rosa, San Clemente, San Nicholas, Santa Barbara, and Santa Catalina Islands comprise the Channel Islands. Ashy storm-petrels occur at their breeding colonies nearly year-round and occur in greater numbers from February through October. The ashy storm-petrel feeds at night on euphausiids, other krill, decapods, larval lanternfish, fish eggs, young squid, and spiny lobster. Previous Federal Actions The purpose of this species report is to provide the best available scientific and commercial information about the species so that we can evaluate whether or not the species warrants protection under the Endangered Species Act of 1973 (Act or ESA). On August 9, 2009, the Service announced its 12-month finding that found, after reviewing the best available scientific and commercial information, listing the ashy storm-petrel was not warranted. The Center for Biological Diversity challenged this decision in the District Court of the Northern District of California on October 25, 2010. -

101 Aizoaceae 1
101 AIZOACEAE 1 Dennis I Morris 2, Marco F Duretto 3 Annual or perennial, monoecious herbs or, less often, shrubs. Leaves opposite or alternate in false whorls, exstipulate or more rarely stipulate, sometimes reduced to scales, often succulent or subsucculent, flat, terete or triquetrous, glabrous, papillose or rarely pubescent or lepidote,. Inflorescence terminal or axillary, flowers solitary or in loose cymes, sessile or pedicellate, bracteate or ebracteate. Flowers usually actinomorphic, bisexual or rarely unisexual and the plant monecious. Sepals (3–)5–8, herbaceous, persistent, often succulent, equal or unequal. Petals 0. Staminodes 0-many, often petaloid and showy in 1–6 whorls (by many authors referred to as petals). Fertile stamens (1–)4–many, free or connate at the base, anthers small. Gynoecium of 2–5(-many) carpels united in a compound ovary, superior, half-inferior or inferior, with as many loculi as carpels; styles as many as loculi; ovules 1-many per locule; placentation usually axile but sometimes parietal or basal. Fruit usually a capsule, dehiscing loculicidally, septicidally, or circumscissile or indehiscent or a berry. Seed with a curved embryo enveloping a mealy endosperm. A family of about 120 genera and 2000–2500 species. Some 100 of the genera earlier considered as constituting the large genus Mesembryanthemum (see Klak et al. 2007). The primary centres of diversity are South Africa and the Mediterranean region. In Australia there are 19 genera and about 80 species, of which 54 species in 8 genera are native. In Tasmania there are 6 genera and 10 species, of which 3 genera and 6 species are intro- duced. -

Guide to Four Easy Veggies You Can Grow
GUIDE TO FOUR EASY VEGGIES YOU CAN GROW Growing and eating homegrown produce is one of the best decisions you could ever make for your health. Homegrown produce is fresher, tastier and often more nutritious than anything you can get at the store. Plus, you’ll reap the health benefits of all that sunshine and exercise outdoors! So let’s start with the basics: Peas, Spinach, Potatoes and Beans are all easy for beginning gardeners to grow. You’ll be off to a great start with these helpful primers, selected as top picks by the editors of MOTHER EARTH NEWS. ALL ABOUT GROWING PEAS by BARBARA PLEASANT Illustrations by KEITH WARD lant peas in mid-spring, when the soil is still cool. The plants Pgrow best in temperatures between 55 and 75 degrees, and young seedlings even tolerate frost. Bacteria growing on pea roots can produce most of the nitrogen the plants need when the crop is grown in cool, moist soil with a pH between 6.5 and 7.0. Types to Try Snap peas are eaten whole, and both Shell peas are often called English Vine length varies from one variety the crunchy pod and the peas inside peas, because many fine varieties were to another, and long-vined peas need taste sweet. Snap peas yield more food developed in Great Britain in the 18th a taller trellis than compact varieties. per square foot than the other types. century. Sweet green peas are shelled Both compact and long-vined va- Snow peas produce tender, flat pods from tough, inedible pods. -

Ferns and Fern Allies
Vascular Plants of Fort Ross State Historic Park August, 2000 Revised April 2020 Rank / Botanical Name Common Name Family ( * = non-native to area, bold = sensitive species) Ferns and Fern Allies Azollaceae/Mosquito Fern Azolla filiculoides Mosquito Fern Blechnaceae/Deer Fern Struthiopteris spicant (Blechnum spicant) Deer Fern Woodwardia fimbriata Giant Chain Fern Dennstaedtiaceae/Bracken Pteridium aquilinum var. pubescens Bracken, Brake Dryopteridaceae/Wood Fern Athyrium filix-femina var.cyclosorum Western Lady Fern Dryopteris arguta Coastal Wood Fern Dryopteris expansa Spreading Wood Fern Polystichum munitum Western Sword Fern Equisetaceae/Horsetail Equisetum arvense Common Horsetail Equisetum hyemale ssp.affine Common Scouring Rush Equisetum laevigatum Smooth Scouring Rush Equisetum telmateia ssp. braunii Giant Horsetail Polypodiaceae/Polypody Polypodium californicum California Polypody Polypodium glycyrrhiza Licorice Fern Polypodium scouleri Leather-leaf Fern Pteridaceae/Brake Adiantum aleuticum Five-finger Fern Adiantum jordanii California Maidenhair Pentagramma triangularis Goldback Fern Gymnosperms/Conifers Cupressaceae/Cypress Hesperocyparis macrocarpa (Cupressus m.) * Monterey Cypress Pinaceae/Pine Abies grandis Grand Fir Pinus lambertiana Sugar Pine Pinus muricata Bishop Pine Pseudotsuga menziesii var. menziesii Douglas-fir Tsuga heterophylla Western Hemlock Taxaceae/Yew Torreya californica California Nutmeg Taxodiaceae/Bald Cypress Sequoia sempervirens Coast Redwood P a g e 1 | 13 Eudicots/Angiosperms/Dicots Aceraceae/Maple -

Inhibition of Lipopolysaccharide-Stimulated Neuro- Inflammatory Kuntze in BV-2 Microglial Cell Mediators by Tetragonia Tetragonoides (Pall)
Lee & Kang Tropical Journal of Pharmaceutical Research December 2014; 13 (12): 2005-2010 ISSN: 1596-5996 (print); 1596-9827 (electronic) © Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. All rights reserved. Available online at http://www.tjpr.org http://dx.doi.org/10.4314/tjpr.v13i12.8 Original Research Article Inhibition of Lipopolysaccharide-Stimulated Neuro- Inflammatory Kuntze in BV-2 Microglial Cell Mediators by Tetragonia tetragonoides (Pall) Sung-Gyu Lee and Hyun Kang* Department of Medical Laboratory Science, College of Health Science, Dankook University, Cheonan-si, Chungnam, 330-714, Republic of Korea *For correspondence: Email: [email protected], [email protected]; Tel: 82-41-550-1452; Fax: 82-41-559-7934 Received: 29 September 2014 Revised accepted: 12 November 2014 Abstract Purpose: To investigate the in vitro antioxidant and anti-neuroinflammatory effects of Tetragonia tetragonoides (Pall.) Kuntze extract (TKE) in lipopolysaccharide (LPS)-stimulated BV-2 microglial cells. Methods: To evaluate the effects of TKE, LPS-stimulated BV microglia were used and the expression and production of inflammatory mediators, namely, nitric oxide (NO), inducible NO synthase (iNOS) and tumor necrosis alpha (TNF-α) were evaluated. Antioxidant activity of TKE was measured using 1, 1- diphenyl-2-picryl-hydrazyl (DPPH) assay. Cell viabilities were estimated by 3-(4, 5-dimethylthiazol-2-yl)- 2, 5- diphenyl-tetrazolium bromide (MTT) assay. Results: TKE significantly suppressed LPS-induced production of NO (p < 0.001 at 20, 40, 80 and 100 μg/ml) and expression of iNOS in BV-2 cells. TKE also suppressed LPS-induced increase in TNF-α level (p < 0.001at 100 μg/ml) in BV-2 cells. -

Fie Cljulhaccin C.Ran9eranct Csociety TOWARDS a BETTER UNDERSTANDING of ANIMAL NUTRITION in PASTORAL SOUTH AUSTRALIA
PROCEEDINGS OF THE AUSTRALIAN RANGELAND SOCIETY BIENNIAL CONFERENCE Official publication of The Australian Rangeland Society Copyright and Photocopying © The Australian Rangeland Society 2012. All rights reserved. For non -personal use, no part of this item may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without prior permission of the Australian Rangeland Society and of the author (or the organisation they work or have worked for). Permission of the Australian Rangeland Society for photocopying of articles for non -personal use may be obtained from the Secretary who can be contacted at the email address, rangelands.exec @gmail.com For personal use, temporary copies necessary to browse this site on screen may be made and a single copy of an article may be downloaded or printed for research or personal use, but no changes are to be made to any of the material. This copyright notice is not to be removed from the front of the article. All efforts have been made by the Australian Rangeland Society to contact the authors. If you believe your copyright has been breached please notify us immediately and we will remove the offending material from our website. Form of Reference The reference for this article should be in this general form; Author family name, initials (year). Title. In: Proceedings of the nth Australian Rangeland Society Biennial Conference. Pages. (Australian Rangeland Society: Australia). For example: Anderson, L., van Klinken, R. D., and Shepherd, D. (2008). Aerially surveying Mesquite (Prosopis spp.) in the Pilbara. -
Kokihi Handout
Tahuri Whenua Inc. Soc. P O Box 1458, Palmerston North. - www.tahuriwhenua.org.nz Kōkihi (New Zealand Spinach – Tetragonia tetragonoides) PRODUCTION NOTES Kōkihi is a plant native to New Zealand, Australia and some parts of South America. It was utilised by Captain James Cook during his contact with Aotearoa/New Zealand in the eighteenth century because of its high Vitamin C content and value against scurvy. While this plant is not related to true spinach, kōkihi is utilised in the same way and has a slightly milder flavour. It is said to be the only true vegetable that Australasia has contributed to the international diet. Kōkihi grows best in warm and dry conditions and is more prevalent in coastal areas throughout New Zealand. It also tolerates salt air well. SOIL PREPARATION Cultivate the seedbed so a fine texture is achieved. Prefers soil pH 6.0 – 7.0 Apply pre-emergence herbicide if preferred. Apply N:P:K fertiliser (30:30:20 for main crops) as a base dressing at planting. Nitrogen (e.g. Urea [40:0:0]) at 40 days after sowing PLANTING Plant mid-late October – early November after frosts and soil temperatures increase (15°C minimum). Soak seeds in cold water for 24 hours before planting to assist germination Plant directly into the prepared ground Spacing between rows should be 1 metre; 40-50cm between plants in the row. Emergence time – 5-28 days, 9 days optimum Seedlings can be grown under plastic/glass and transplanted at the 4-6 leaf stage Requires 25000/ha CROP MANAGEMENT A base dressing of fertiliser is used at planting then a nitrogen fertiliser at approximately 30-40 days is all that is required for crop establishment.