Xa9949546 3. Reactor Pressure Vessel Materials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Suzuki Announces FY2019 Vehicle Recycling Results in Japan
22 June 2020 Suzuki Announces FY2019 Vehicle Recycling Results in Japan Suzuki Motor Corporation has today announced the results of vehicle recycling for FY2019 (April 2019 to March 2020) in Japan, based on the Japan Automobile Recycling Law*1. In line with the legal mandate, Suzuki is responsible for promoting appropriate treatment and recycling of automobile shredder residue (ASR), airbags, and fluorocarbons through recycling fee deposited from customers. Recycling of these materials are appropriately, smoothly, and efficiently conducted by consigning the treatment to Japan Auto Recycling Partnership as for airbags and fluorocarbons, and to Automobile Shredder Residue Recycling Promotion Team*2 as for ASR. The total cost of recycling these materials was 3,640 million yen. Recycling fees and income generated from the vehicle-recycling fund totalled 4,150 million yen, contributing to a net surplus of 510 million yen. For the promotion of vehicle recycling, Suzuki contributed a total of 370 million yen from the above net surplus, to the Japan Foundation for Advanced Auto Recycling, and 20 million yen for the advanced recycling business of the Company. For the mid-and long-term, Suzuki continues to make effort in stabilising the total recycling costs. Moreover, besides the recycling costs, the Company bears 120 million yen as management-related cost of Japan Automobile Recycling Promotion Center and recycling-related cost of ASR. The results of collection and recycling of the materials are as follows. 1. ASR - 60,388.3 tons of ASR were collected from 450,662 units of end-of-life vehicles - Recycling rate was 96.7%, exceeding the legal target rate of 70% set in FY2015 since FY2008 2. -

読む Notice of the 89Th Annual General Meeting of Shareholders
Note: This document has been translated from a part of the Japanese original for reference purposes only. In the event of any discrepancy between this translated document and the Japanese original, the original shall prevail. The Company assumes no responsibility for this translation or for direct, indirect or any other forms of damages arising from the translation. (Stock Exchange Code 5631) June 1, 2015 To Shareholders with Voting Rights: Ikuo Sato Representative Director & President The Japan Steel Works, Ltd. 11-1, Osaki 1-chome, Shinagawa-ku, Tokyo, Japan NOTICE OF THE 89TH ANNUAL GENERAL MEETING OF SHAREHOLDERS Dear Shareholders: We would like to express our appreciation for your continued support and patronage. You are cordially invited to attend the 89th Annual General Meeting of Shareholders of The Japan Steel Works, Ltd. (the “Company”). The meeting will be held for the purposes as described below. If you are unable to attend the meeting, you can exercise your voting rights in writing or via the Internet. Please review the attached Reference Documents for the General Meeting of Shareholders and exercise your voting rights by 5:00 p.m. on Tuesday, June 23, 2015, Japan time. If exercising your voting rights in writing, indicate your vote for or against the proposal on the enclosed Voting Rights Exercise Form and return it so that it is received by the above-mentioned deadline. If exercising your voting rights via the Internet, visit the Company’s Web site (http://www.web54.net) to exercise your voting rights, enter the voting rights exercise code and password that are indicated on the enclosed Voting Rights Exercise Form, follow the instructions on the screen and input your vote for or against each proposal by the above-mentioned deadline. -

Toyota, Suzuki to Work Together in Green, Safety Technology 6 February 2017, by Yuri Kageyama
Toyota, Suzuki to work together in green, safety technology 6 February 2017, by Yuri Kageyama other with products and components. The next step would be to come up with specific cooperation projects, they said. Suzuki does not have a hybrid, electric car or fuel cell vehicle in its lineup. Self-driving cars are also a growing focus in the industry. Toyota President Akio Toyoda praised Suzuki's pioneer spirit. "I am truly thankful for having been given this opportunity to work together with a company such as Suzuki, which overflows with the spirit of In this Oct. 12, 2016 file photo, Toyota Motor Corp. challenge. Toyota looks forward to learning much," President Akio Toyoda, left, speaks with Suzuki Motor he said in a statement. Corp. Chairman Osamu Suzuki during a news conference in Tokyo. Japanese automakers Toyota and Developing futuristic technology is costly, and the Suzuki, which began looking into a partnership in automakers can hope to reduce costs by working October, say they have decided to work together in together. Toyota and Suzuki have encouraged ecological and safety technology—a rapidly growing area others to join the partnership. in the industry. Toyota Motor Corp., the maker of the Camry sedan, Prius hybrid and Lexus luxury models, "We now stand at the starting point for building a and Suzuki Motor Corp., which specializes in tiny cars, announced the decision Monday, Feb. 6, 2017, following concrete cooperative relationship. I want to give approval by the company boards. (Shigeyuki this effort our fullest and to aim at producing results Inakuma/Kyodo News via AP, File) that will lead Toyota to conclude that it was the right thing for Toyota to have decided to work together with Suzuki," said Suzuki Chairman Osamu Suzuki. -

Takao Suzuki and Vice President, Scott Henderson Set Direction for 2020
Qually T Welcomes Associates Back from Shutdown Honda Transmission Mfg. of America, Inc. INSIDE THIS EDITION 3 Executive New Years Address HTM President, Mr. Takao Suzuki and Vice President, Scott Henderson set direction for 2020. Takao Suzuki Scott Henderson 9 HTM President HTM Vice President Biometric Screenings Scheduling your biometric screening. 11 15 Honoring Dr. Martin Luther King, Jr. HTM and AEP associates honoring the Leadership Insights ATM Division Manager, Greg Dawson Legacy of Dr. Martin Luther King, Jr. at shares his insight on the importance of The Annual MLK Breakfast in Lima Ohio. operating with ethics and integrity. 1 INSIDE THIS EDITION 19 HTM Activity Center Meet the staff and discover what 17 offerings they have to help you improve HTM’s Associate Relations Group your overall health. Meet the HTM Associate Relations (AR) Group. 21 22 From our kitchen to your kitchen. This quarters recipe from AVI’s Mark & Sherri.. E-Learning Introducing OpenSesame eLearning 25 Environmental message from HTM Executives 2 Executive 2020 New Years Address Takao Suzuki Scott Henderson HTM President HTM Vice President As associates returned from shutdown and the year 2020 began, HTM President, Takao Suzuki and Vice President, Scott Henderson greeted them with their annual New Years Address. Mr. Suzuki began by reviewing HTM’s accomplishments from 2019. He noted that, “mass production of Co-ax Transfer started in 2018 and continued to stabilize in 2019 and production of the FH3 began and good quality has been maintained throughout 2019. This is a result of each associates hard work”. He went on to say, “because business conditions are changing faster and in greater scale than ever before, we must accommodate changes and adjust production plans as well as business plans on a daily basis. -

Whither the Keiretsu, Japan's Business Networks? How Were They Structured? What Did They Do? Why Are They Gone?
IRLE IRLE WORKING PAPER #188-09 September 2009 Whither the Keiretsu, Japan's Business Networks? How Were They Structured? What Did They Do? Why Are They Gone? James R. Lincoln, Masahiro Shimotani Cite as: James R. Lincoln, Masahiro Shimotani. (2009). “Whither the Keiretsu, Japan's Business Networks? How Were They Structured? What Did They Do? Why Are They Gone?” IRLE Working Paper No. 188-09. http://irle.berkeley.edu/workingpapers/188-09.pdf irle.berkeley.edu/workingpapers Institute for Research on Labor and Employment Institute for Research on Labor and Employment Working Paper Series (University of California, Berkeley) Year Paper iirwps-- Whither the Keiretsu, Japan’s Business Networks? How Were They Structured? What Did They Do? Why Are They Gone? James R. Lincoln Masahiro Shimotani University of California, Berkeley Fukui Prefectural University This paper is posted at the eScholarship Repository, University of California. http://repositories.cdlib.org/iir/iirwps/iirwps-188-09 Copyright c 2009 by the authors. WHITHER THE KEIRETSU, JAPAN’S BUSINESS NETWORKS? How were they structured? What did they do? Why are they gone? James R. Lincoln Walter A. Haas School of Business University of California, Berkeley Berkeley, CA 94720 USA ([email protected]) Masahiro Shimotani Faculty of Economics Fukui Prefectural University Fukui City, Japan ([email protected]) 1 INTRODUCTION The title of this volume and the papers that fill it concern business “groups,” a term suggesting an identifiable collection of actors (here, firms) within a clear-cut boundary. The Japanese keiretsu have been described in similar terms, yet compared to business groups in other countries the postwar keiretsu warrant the “group” label least. -

SUV Fit Guide
SUV Fit Guide Size Years Vehicle 98-98 Chevy Tracker 2dr 99-04 Chevy Tracker 2dr 89-97 Geo Tracker 2dr 86-95 Suzuki Samurai 89-98 Suzuki Sidekick 2dr 99-04 Suzuki Vitara 2dr Extra Small 96-99 Toyota RAV4 2dr Size Years Vehicle Years Vehicle 05-09 BMW X3 55-86 Jeep CJ SUV * 95-05 Chevy Blazer 2-door 07-09 Jeep Compass 83-94 Chevy S10 Blazer 02-09 Jeep Liberty 98-98 Chevy Tracker 4dr * 07-09 Jeep Patriot 99-04 Chevy Tracker 4dr * 87-09 Jeep Wrangler * 07-09 Dodge Nitro 04-09 Jeep Wrangler Unlimited 01-09 Ford Escape 95-09 Kia Sportage * 96-97 Geo Tracker 4dr * 94-97 Land Rover Defender 90 92-94 GMC Jimmy 02-05 Land Rover Freelander 95-99 GMC Jimmy 2-door 08-09 Land Rover LR2 Small 83-91 GMC S15 Jimmy 01-09 Mazda Tribute 92-93 GMC Typhoon 05-09 Mercury Mariner 97-09 Honda CR-V * 91-94 Oldsmobile Bravada 05-09 Hyundai Tucson 99-09 Suzuki Grand Vitara * 89-00 Isuzu Amigo 99-04 Suzuki Vitara 4dr * 01-03 Isuzu Rodeo 2dr 96-05 Toyota RAV4 4dr * 99-01 Isuzu VehiCROSS * 09-09 Volkswagen Tiguan 84-01 Jeep Cherokee Size Years Vehicle Years Vehicle 07-09 Acura RDX 03-09 Kia Sorento 00-06 BMW X5 94-04 Land Rover Discovery 95-05 Chevy Blazer 4-door 99-03 Lexus RX300 99-01 Chevy Blazer Trailblazer 07-09 Mazda CX-7 66-77 Ford Bronco * 91-94 Mazda Navajo 84-90 Ford Bronco II * 98-05 Mercedes-Benz M-Class 91-03 Ford Explorer 2dr 87-04 Nissan Pathfinder 98-00 GMC Envoy 08-09 Nissan Rogue 95-01 GMC Jimmy 4-door 00-09 Nissan Xterra 94-02 Honda Passport 96-04 Oldsmobile Bravada Medium 01-06 Hyundai Santa Fe 01-05 Pontiac Aztek 08-09 Infiniti EX 02-09 Saturn -
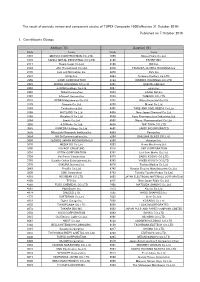
Published on 7 October 2016 1. Constituents Change the Result Of
The result of periodic review and component stocks of TOPIX Composite 1500(effective 31 October 2016) Published on 7 October 2016 1. Constituents Change Addition( 70 ) Deletion( 60 ) Code Issue Code Issue 1810 MATSUI CONSTRUCTION CO.,LTD. 1868 Mitsui Home Co.,Ltd. 1972 SANKO METAL INDUSTRIAL CO.,LTD. 2196 ESCRIT INC. 2117 Nissin Sugar Co.,Ltd. 2198 IKK Inc. 2124 JAC Recruitment Co.,Ltd. 2418 TSUKADA GLOBAL HOLDINGS Inc. 2170 Link and Motivation Inc. 3079 DVx Inc. 2337 Ichigo Inc. 3093 Treasure Factory Co.,LTD. 2359 CORE CORPORATION 3194 KIRINDO HOLDINGS CO.,LTD. 2429 WORLD HOLDINGS CO.,LTD. 3205 DAIDOH LIMITED 2462 J-COM Holdings Co.,Ltd. 3667 enish,inc. 2485 TEAR Corporation 3834 ASAHI Net,Inc. 2492 Infomart Corporation 3946 TOMOKU CO.,LTD. 2915 KENKO Mayonnaise Co.,Ltd. 4221 Okura Industrial Co.,Ltd. 3179 Syuppin Co.,Ltd. 4238 Miraial Co.,Ltd. 3193 Torikizoku co.,ltd. 4331 TAKE AND GIVE. NEEDS Co.,Ltd. 3196 HOTLAND Co.,Ltd. 4406 New Japan Chemical Co.,Ltd. 3199 Watahan & Co.,Ltd. 4538 Fuso Pharmaceutical Industries,Ltd. 3244 Samty Co.,Ltd. 4550 Nissui Pharmaceutical Co.,Ltd. 3250 A.D.Works Co.,Ltd. 4636 T&K TOKA CO.,LTD. 3543 KOMEDA Holdings Co.,Ltd. 4651 SANIX INCORPORATED 3636 Mitsubishi Research Institute,Inc. 4809 Paraca Inc. 3654 HITO-Communications,Inc. 5204 ISHIZUKA GLASS CO.,LTD. 3666 TECNOS JAPAN INCORPORATED 5998 Advanex Inc. 3678 MEDIA DO Co.,Ltd. 6203 Howa Machinery,Ltd. 3688 VOYAGE GROUP,INC. 6319 SNT CORPORATION 3694 OPTiM CORPORATION 6362 Ishii Iron Works Co.,Ltd. 3724 VeriServe Corporation 6373 DAIDO KOGYO CO.,LTD. 3765 GungHo Online Entertainment,Inc. -

Nissan Multikit 2005-2015 Suzuki Equator 2009
99-7635 INSTALLATION INSTRUCTIONS Nissan Multikit 2005-2015 TABLE OF CONTENTS See page 2 Suzuki Equator 2009 KIT FEATURES WIRING & ANTENNA CONNECTIONS (sold separately) • ISO DIN radio provision with pocket Wiring Harness: Please visit metraonline.com • ISO DDIN radio provision for options Antenna Adapter: Please visit metraonline.com for options KIT COMPONENTS • A) Radio housing • B) Radio brackets • C) Pocket • D) (4) #8 x 3/8” Phillips screws TOOLS REQUIRED • Phillips screwdriver • Panel removal tool A B C D CAUTION! All accessories, switches, climate controls panels, and especially air bag indicator lights must be connected before cycling the ignition. Also, do not remove the factory radio with the key in the on position, or while the vehicle is running. Metra. The World’s Best Kits.® MetraOnline.com © COPYRIGHT 2018 METRA ELECTRONICS CORPORATION REV. 2/28/18 INST99-7635 APPLICATIONS TABLE OF CONTENTS Nissan Suzuki Dash Disassembly Kit Assembly Frontier LE ....................................... 2005-2008 Equator ..................................................... 2009 Nissan – ISO DIN radio provision with pocket ...........5 Frontier S ............................................2011-2012 – Pathfinder 2005-2012 ...................................3 – ISO DDIN radio provision ..............................6 Frontier S (king cab) ......................... 2013-2015 Frontier SE ....................................... 2005-2008 – Frontier 2005-2015 .......................................4 Frontier SE (4-cylinder engine) ......2009-2011 -

Annual Report 2015
Network Düsseldorf Corona Detroit New York (near Los Angeles) Gurgaon Shanghai Houston (near Delhi) Shenzhen Taipei Hanoi Hong Kong Bangkok Manila Kuala Lumpur Singapore Jakarta Domestic Head Oice Hiroshima Oice Muroran Plant Gate City Ohsaki-West Tower, 11-1, Osaki 1-chome, 6-1, Funakoshi-Minami 1-chome, Aki-ku, 4, Chatsumachi, Muroran-shi, Shinagawa-ku, Tokyo 141-0032, Japan Hiroshima-shi, Hiroshima 736-8602, Japan Hokkaido 051-8505, Japan Phone: +81-3-5745-2001 Facsimile: +81-3-5745-2025 Phone: +81-82-822-0991 Facsimile: +81-82-822-0997 Phone: +81-143-22-0143 Facsimile: +81-143-24-3440 Nagoya Oice Fukuoka Oice Hiroshima Plant Mitsui Sumitomo Kaijo Nagoya Shirakawa Bldg. 7F, 9-15, 23-2, Sakuragaoka 1-chome, Kasuga-shi, Fukuoka 6-1, Funakoshi-Minami 1-chome, Aki-ku, Sakae 2-chome, Naka-ku, Nagoya-shi, Aichi 460-0008, Japan 816-0872, Japan Hiroshima-shi, Hiroshima 736-8602, Japan Phone: +81-52-222-1271 Facsimile: +81-52-222-1275 Phone: +81-92-582-8111 Facsimile: +81-92-582-8124 Phone: +81-82-822-3181 Facsimile: +81-82-285-2038 Osaka Oice Yokohama Plant Shinanobashi Mitsui Bldg., 11-7, Utsubohonmachi 2-1, Fukuura 2-chome, Kanazawa-ku, 1-chome, Nishi-ku, Osaka-shi, Osaka 550-0004, Japan Yokohama-shi, Kanagawa 236-0004, Japan Phone: +81-6-6446-2480 Facsimile: +81-6-6446-2488 Phone: +81-45-781-1111 Facsimile: +81-45-787-7200 Overseas Japan Steel Works America, Inc. JSW Plastics Machinery (TAIWAN) Corp. NINGBO TONGYONG PLASTIC MACHINERY Head Oice Head Oice MANUFACTURING CO., LTD. 122 East 42nd Street, Suite 3810 New York, NY 10168, USA 1F., No. -

Mazda North American Operations (U-63-10-0535) Powertrain Engineering Group 46976 Magellan Drive Wixom, Michigan 48393
MACES- Activity Report Page 1 of 4 DEPARTMENT OF ENVIRONMENTAL QUALITY AIR QUALITY DIVISION ACTIVITY REPORT: On-site Inspection U6310053555433 FACILITY: Mazda North American Operations SRN / ID: U63100535 LOCATION: 46976 Magellan Drive, Wixom DISTRICT: Warren CITY: Wixom COUNTY: OAKLAND CONTACT: ACTIVITY DATE: 09/21/2020 STAFF: Iranna Konanahalli I COMPLIANCE STATUS: Compliance SOURCE CLASS: SUBJECT: FY 2020 inspection of Mazda North American Operations (“Mazda”) RESOLVED COMPLAINTS: Mazda North American Operations (U-63-10-0535) Powertrain Engineering Group 46976 Magellan Drive Wixom, Michigan 48393- SRN reassign: Suzuki Tech Center (U-63-10-0535) è Mazda North American Operations (U-63-10-0535). Mazda moved to Wixom in 2013. Permit-to-Install: Based upon AQD’s interim policy (June 12, 2013, AQD Chief Hellwig e-mail), at the option of the company, AQD will issue Rule 201 permit for Chassis Dynamometers, if requested. Not subject to (not major source for HAPs): Major Source NESHAP / MACT 5P, 40 CFR Part 63, Subpart PPPPP, National Emission Standards for Hazardous Air Pollutants: Engine Test Cells/Stands, (Page 28774, Federal Register / Vol. 68, No. 101 / Tuesday, May 27, 2003 / Rules and Regulations) / Final rule. Not Subject to: NESHAP/ MACT T, area source National Emission Standards for Hazardous Air Pollutants: Halogenated Solvent Cleaning (40 CFR, Part 63, Subpart T; NESHAP/ MACT T); Correction; 29484 Federal Register / Vol. 60, No. 107 / Monday, June 5, 1995 / Rules and Regulations; amended National Air Emission Standards for Hazardous Air Pollutants: Halogenated Solvent Cleaning (40 CFR, Part 63, Subpart T); Final Rule; Page 25138 Federal Register / Vol. 72, No. 85 / Thursday, May 3, 2007 / Rules and Regulations. -

Annual Report 2017 for the Year Ended March 31, 2017 from a Company Making Things, to a Company Making People Smile
Annual Report 2017 For the year ended March 31, 2017 From a company making things, to a company making people smile. Just a glance at our vehicles and you can imagine days filled with excitement. Just a glimpse of jet wings above and you can envision worlds yet unseen. But no matter the time or place, we are always by our customers’ sides. What is important is not how many cars we make, but how many smiles we can create. More than a century has passed since the founding of Aircraft Research Laboratory, the forerunner of SUBARU. Now, Fuji Heavy Industries Ltd. has been reborn as SUBARU CORPORATION. From making things to delivering value that shines in people’s hearts, SUBARU aims to touch the hearts of people and bring smiles to their faces. We constantly challenge ourselves to ensure that satisfaction with SUBARU is reflected in the happy faces of our customers. SUBARU CORPORATION 01 ANNUAL REPORT 2017 SUBARU CORPORATION 02 ANNUAL REPORT 2017 Vision for 2020 Not big in size, but a high-quality company with distinctive strengths For SUBARU, by no means a large automaker, the two strategies of uncompromising differentiation and added-value are essential for achieving sustained growth in a fierce competitive environment. We will narrow our focus to categories and markets in which we can leverage our strengths, practice selection and concentration in allocation of limited management resources, and further accelerate two initiatives: enhancing the SUBARU brand and building a strong business structure. And, we will seek to have a prominent presence in customers’ minds. -

CO2 Emissions from New Passenger Cars in Europe: Car Manufacturers’ Performance in 2019
BRIEFING © 2020 INTERNATIONAL COUNCIL ON CLEAN TRANSPORTATION AUGUST 2020 CO2 emissions from new passenger cars in Europe: Car manufacturers’ performance in 2019 Prepared by: Uwe Tietge, Peter Mock, Jan Dornoff This briefing paper provides an overview of CO2 emission levels of new passenger cars in the European Union (EU) in 2019 based on a preliminary dataset recently released by the European Environment Agency (EEA).1 The dataset showed that new cars sold in the EU in 2019 had average CO2 emissions of 122 g CO2/km, 1 g/km higher than in 2018, as measured over the New European Driving Cycle (NEDC). As a follow-up to the previous year’s briefing,2 this paper details manufacturer performance in terms of CO2 emissions reduction, fuel type and technology trends, and market share. The paper focuses on differences between Member States, as well as between the major car makers. It also discusses flexible compliance mechanisms and presents data on the Worldwide Harmonized Light Vehicles Test Procedure (WLTP). The preliminary EEA dataset used in this briefing has yet to be validated. The final dataset will be published at the end of 2020, so the specific values used in this report may change. The preliminary data for 2019 should, however, provide relatively reliable results.3 The ICCT will review the final European emissions data in the next edition of the European Vehicle Market Statistics Pocketbook.4 www.theicct.org 1 European Environment Agency, “Monitoring of CO2 Emissions from Passenger Cars – Regulation (EU) 2019/631 —European Environment Agency,” Data, June 26, 2020, https://www.eea.europa.eu/data-and-maps/data/co2- [email protected] cars-emission-18.