Reaction of Tangerines Genotypes to Elsinoe Fawcettiiunder
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cultivar and Rootstock Research for the Arizona Citrus Industry
Supporting the University of Arizona Citrus Variety Collection 20171 Glenn C. Wright2 2School of Plant Sciences, University of Arizona, Yuma Agriculture Center, Yuma, AZ Introduction The 8-acre citrus variety collection at the Yuma Agriculture Center is the most comprehensive collection of citrus within Arizona, containing about 110 selections. The collection was propagated in 1993 and is found in Block 17. The collection has value because it serves as a resource for research, teaching and extension. From 1993 until 2008, data was taken from the lemon selections within the collection. We use fruit, leaves and flowers from the collection to teach students of the Citrus and Date palm production course that I teach through UA-Yuma. A part of one laboratory session takes place within the collection. We also use the collection to teach Master Gardeners, and the we lead tours through the collection for Master Gardeners and other interested parties. We use fruit from the collection for displays and tasting for community events. Now, the trees are aging, and we are in the process of re-propagating the collection into a 3-acre parcel in Block 18 at the Center, but the task is not yet finished. Some of those new trees are planted in the new blocks while others are growing in a field nursery and others have yet to be budded. About one acre of the new collection is in the ground. Few of the new trees have fruit. The University has borne the cost of maintaining the collection for the past 23 years, but those costs can no longer be absorbed. -

Observations on Impietratura Disease Symptoms in Four Citrus Species
Observations on Impietratura Disease Symptoms in Four Citrus Species A. Caruso, M. Davino and G. Terranova ABSTRACT. Citrus impietratura disease affects citrus cultivars in the Mediterranean Basin. The indexing of impietratura disease is based on symptoms on indicator plants such as Volkamer lemon or grapefruit or on the inspection of the trees in the field. This requires many years. In this paper the effects of a severe isolate of impietratura on sweet orange, Volkamer lemon, rough lemon and Marsh Seedless grapefruit is reported. Our observations indicate that rough lemon is a better indicator of fruit symptoms than Volkamer lemon, grapefruit and sweet orange. Index words. biological assay, albedo gumming, rough lemon. In Italy cristacortis, concave gum servation for at least three years. Dif- and impietratura are widespread in ficulties in timely indexing of old line commercial orchards. The causal Navelina trees planted in Italy be- agents of these diseases have not been tween 1970 and 1985 resulted in the characterized and their detection is high percentage of infection (>30%) by still by means of biological assays. impietratura, concave gum and Due of its excellent bioagronomic psorosis A (4,6,13). and marketing character, in Italy the Rough lemon was initially consid- cultivation of the Navelina sweet ered to be tolerant to impietratura dis- orange spread rapidly in many citrus- ease (9), but later studies showed it to growing areas by growers, despite the be susceptible (7,8,12) and Catara and fact that the first trees imported pre- Scaramuzzi (5) suggested its use as an sented very mild flecking symptoms on alternative indicator. -

Soluble Solids Accumulation in ʻvalenciaʼ Sweet Orange As Related to Rootstock Selection and Fruit Size
J. AMER. SOC. HORT. SCI. 129(4):594–598. 2004. Soluble Solids Accumulation in ʻValenciaʼ Sweet Orange as Related to Rootstock Selection and Fruit Size Graham H. Barry1 and William S. Castle Citrus Research and Education Center, University of Florida, 700 Experiment Station Road, Lake Alfred, FL 33850-2299 Frederick S. Davies Department of Horticultural Sciences, University of Florida, P.O. Box 110690, Gainesville, FL 32611-0690 ADDITIONAL INDEX WORDS. Citrus sinensis, juice quality, soluble solids concentration (SSC) ABSTRACT. Juice quality of ʻValenciaʼ sweet orange [Citrus sinensis (L.) Osb.] trees on Carrizo citrange [C. sinensis x Poncirus trifoliata (L.) Raf.] or rough lemon (C. jambhiri Lush.) rootstocks was determined for fruit harvested by canopy quadrant and separated into size categories to ascertain the direct role of rootstock selection on juice soluble solids concentration (SSC) and soluble solids (SS) production per tree of citrus fruit. SS production per fruit and per tree for each size category was calculated. Juice quality was dependent on rootstock selection and fruit size, but independent of canopy quadrant. Fruit from trees on Carrizo citrange had >20% higher SSCs than fruit from trees on rough lemon, even for fruit of the same size. Large fruit accumulated more SS per fruit than smaller fruit, despite lower juice content and SSC. Within rootstocks, SS content per fruit decreased with decreasing fruit size, even though SSC increased. Rootstock effect on juice quality was a direct rather than an indirect one mediated through differences in fruit size. The conventional interpretation of juice quality data that differences in SSC among treatments, e.g., rootstocks or irrigation levels, or fruit size, are due to “dilution” of SS as a result of differences in fruit size and, hence, juice volume, is only partly supported by these data. -

Kumquats Are Tiny Citrus to Use in Relish Or Marmalade
KUMQUATS ARE TINY CITRUS TO USE IN RELISH OR MARMALADE In response to numerous inquiries about kumquats, which are available in various quantities from November through June, suggestions are offered here for using and preserving the unusual fruit. The kumquat is a small orange fruit of the citrus family. It is oblong and about the size of a small plum; the rind is golden-orange, the flesh is rather dry and the seeds are small. When fully ripe, kumquats can be eaten raw— cut up or sliced and added to salads and fruit cups. More often they are enjoyed cooked whole in a sugar syrup, candied, or in marmalade. Clip these recipes for your file. Preserved Kumquats 2 cups sugar 2 quarts firm kumquats Wash kumquats. Pour over them boiling water to 5 cups sugar cover. Let them steep 2 minutes, then pour off the water. Stick 1 whole clove into each kumquat. Make Remove stems and leaves from kumquats. Wash and a syrup of the water and sugar. Bring to a boil. drain the fruit. Prick each kumquat several times Poach kumquats in the syrup for about 20 minutes, or with a darning needle. Put the fruit into a saucepan until tender. and cover with boiling water. Simmer for about 20 minutes or until tender. Candied Kumquats Skim the kumquats from the water and stir into the 1 quart whole kumquats (about 1 pound) water the 5 cups sugar. Boil this syrup 5 minutes. 2 cups sugar Add the kumquats and cook them gently for about 1 1 cup water hour, or until the fruit is transparent. -

Canker and Greening – Lessons from South America by Bob Rouse and Fritz Roka
Citrus Expo follow-up Canker and greening – lessons from South America By Bob Rouse and Fritz Roka he 2006 Citrus Expo featured citrus managers from Cecil Taylor described citrus production in the northeast Brazil and Argentina who spoke about their manage - region of Argentina where producers grow common oranges, ment programs to control citrus canker and citrus mandarins and grapefruit. Tgreening diseases. The strategies they described Argentina was devastated by tristeza in the 1940s and in have been successful and hopefully transferable to citrus the 1960s began to see canker. Initial control efforts included production in Florida. eradication, tree defoliation and spraying enough copper that The Florida canker eradication program was abandoned in trees turned blue. These efforts proved fruitless, and growers the spring of 2006 after the USDA predicted that canker demanded an end to any eradication policy. would spread by 100,000 acres before the end of the year. During these early years, windbreaks were not part of any Currently, most of the canker is south of Polk County’s State perceived solution. By the end of the 1980s, they began to re - Road 60. Since the eradication program ended, there have alize, however, that the key to canker control lies in slowing been about 100 new finds monthly. down the wind, even though they did not experience strong Grower self-inspections have accounted for 40 percent of winds. They started with low-growing plants like sugarcane, the new finds. DPI inspectors have identified the remaining maze and sunflower. By the 1990s, they saw the need for 60 percent of new canker finds. -

Rangpur Lime X Troyer Citrange, a Hybrid Citrus Rootstock for Closely Spaced Trees
Proc. Fla. State Hort. Soc. 99:33-35. 1986. RANGPUR LIME X TROYER CITRANGE, A HYBRID CITRUS ROOTSTOCK FOR CLOSELY SPACED TREES W. S. Castle A combination of diseases, repeated freezes, and other University of Florida, IFAS factors has reemphasized the importance of rootstocks in Citrus Research and Education Center Florida. Moreover, the effects of these factors illustrate the 700 Experiment Station Road inherent weaknesses in virtually all citrus rootstocks and Lake Alfred, FL 33850 the need to continually search for new, improved ones. Another recent trend related to rootstocks has been C. O. YOUTSEY the shift toward more closely spaced trees, particularly FDACS, Division of Plant Industry within the row (7,8). Rootstocks well-suited for dense plan Citrus Budwood Registration Bureau tings have not been available although such stocks are 3027 Lake Alfred Road being evaluated and one appears particularly promising Winter Haven, FL 33881 (1, 3, 4, 8). It is a hybrid of Rangpur lime and Troyer D. J. Hutchison citrange (RxT) and has been under study in Florida for 18 United States Department of Agriculture yr. During this period, trees on RxT have demonstrated Agricultural Research Service sufficient commercial potential to justify our presentation 2120 Camden Road in this report of their performance and a description of Orlando, FL 32803 RxT and its characteristics. Additional index words. Blight, tristeza, tree size control. History Dr. J. R. Furr, formerly a plant breeder with the U.S. Abstract. A hybrid of Rangpur lime (Citrus limonia Osb.) and Department of Agriculture (USDA) at Indio, California, Troyer citrange [ C. sinensis (L) Osb. -

Kaffir Lime and Lemon Cordial Recipe : SBS Food
8/14/2019 Kaffir lime and lemon cordial recipe : SBS Food SBS World Movies Voices Indigenous Untold Australia The Handmaid's Tale FOOD sbs.com.au/food AUSTRALIAN Kaffir lime and lemon cordial 0 This recipe makes plenty, because you’ll want to have it as your drink of choice for a fortnight – it keeps well in the fridge for at least a couple of weeks. I really like the fine texture of the zest, but you can sieve it out if you feel it gets in the way when you drink it. Makes Preparation min10 Skill level Easy Ingredients 4 lemons, scrubbed, zested, juiced, squeezed halves reserved 4 kaffir lime leaves 3 cm-piece ginger, bruised with the back of a knife 700 g caster sugar 50 g tartaric acid ice cubes, to serve Cook's notes Oven temperatures are for conventional; if using fan-forced (convection), reduce the temperature by 20˚C. | We use Australian tablespoons and cups: 1 teaspoon equals 5 ml; 1 tablespoon equals 20 ml; 1 cup equals 250 ml. | All herbs are fresh (unless specified) and cups are lightly packed. | All vegetables are medium size and peeled, unless specified. | All eggs are 55-60 g, unless specified. https://www.sbs.com.au/food/recipes/kaffir-lime-and-lemon-cordial 1/2 8/14/2019 Kaffir lime and lemon cordial recipe : SBS Food Instructions Makes 1.2 L cordial for about 12 L prepared drink Cooling time 15 minutes Infusing time overnight Place lemon zest, juice and squeezed halves, lime leaves, ginger and caster sugar in a large heatproof bowl. -

Known Host Plants of Huanglongbing (HLB) and Asian Citrus Psyllid
Known Host Plants of Huanglongbing (HLB) and Asian Citrus Psyllid Diaphorina Liberibacter citri Plant Name asiaticus Citrus Huanglongbing Psyllid Aegle marmelos (L.) Corr. Serr.: bael, Bengal quince, golden apple, bela, milva X Aeglopsis chevalieri Swingle: Chevalier’s aeglopsis X X Afraegle gabonensis (Swingle) Engl.: Gabon powder-flask X Afraegle paniculata (Schum.) Engl.: Nigerian powder- flask X Atalantia missionis (Wall. ex Wight) Oliv.: see Pamburus missionis X X Atalantia monophylla (L.) Corr.: Indian atalantia X Balsamocitrus dawei Stapf: Uganda powder- flask X X Burkillanthus malaccensis (Ridl.) Swingle: Malay ghost-lime X Calodendrum capense Thunb.: Cape chestnut X × Citroncirus webberi J. Ingram & H. E. Moore: citrange X Citropsis gilletiana Swingle & M. Kellerman: Gillet’s cherry-orange X Citropsis schweinfurthii (Engl.) Swingle & Kellerm.: African cherry- orange X Citrus amblycarpa (Hassk.) Ochse: djerook leemo, djeruk-limau X Citrus aurantiifolia (Christm.) Swingle: lime, Key lime, Persian lime, lima, limón agrio, limón ceutí, lima mejicana, limero X X Citrus aurantium L.: sour orange, Seville orange, bigarde, marmalade orange, naranja agria, naranja amarga X Citrus depressa Hayata: shiikuwasha, shekwasha, sequasse X Citrus grandis (L.) Osbeck: see Citrus maxima X Citrus hassaku hort. ex Tanaka: hassaku orange X Citrus hystrix DC.: Mauritius papeda, Kaffir lime X X Citrus ichangensis Swingle: Ichang papeda X Citrus jambhiri Lushington: rough lemon, jambhiri-orange, limón rugoso, rugoso X X Citrus junos Sieb. ex Tanaka: xiang -

Tropical Horticulture: Lecture 32 1
Tropical Horticulture: Lecture 32 Lecture 32 Citrus Citrus: Citrus spp., Rutaceae Citrus are subtropical, evergreen plants originating in southeast Asia and the Malay archipelago but the precise origins are obscure. There are about 1600 species in the subfamily Aurantioideae. The tribe Citreae has 13 genera, most of which are graft and cross compatible with the genus Citrus. There are some tropical species (pomelo). All Citrus combined are the most important fruit crop next to grape. 1 Tropical Horticulture: Lecture 32 The common features are a superior ovary on a raised disc, transparent (pellucid) dots on leaves, and the presence of aromatic oils in leaves and fruits. Citrus has increased in importance in the United States with the development of frozen concentrate which is much superior to canned citrus juice. Per-capita consumption in the US is extremely high. Citrus mitis (calamondin), a miniature orange, is widely grown as an ornamental house pot plant. History Citrus is first mentioned in Chinese literature in 2200 BCE. First citrus in Europe seems to have been the citron, a fruit which has religious significance in Jewish festivals. Mentioned in 310 BCE by Theophrastus. Lemons and limes and sour orange may have been mutations of the citron. The Romans grew sour orange and lemons in 50–100 CE; the first mention of sweet orange in Europe was made in 1400. Columbus brought citrus on his second voyage in 1493 and the first plantation started in Haiti. In 1565 the first citrus was brought to the US in Saint Augustine. 2 Tropical Horticulture: Lecture 32 Taxonomy Citrus classification based on morphology of mature fruit (e.g. -

The Production and Area of Pomelo in China (1990-2007)
TThhee PPoommeellooIInndduussrrttyy iinn CChhiinnaa Institute of Fruit Tree Research, Guangdong Academy of Agricultural Science, China Yi Ganjun [email protected] 1800 2005 1580 1600 2006 2007 ) 1400 T 0 0 1200 0 1 × ( . 1000 n o i t 800 c u d 547 o r 600 P 390 430 400 245 290 175 176 178 182 200 0 Cuba Argentina India Turkey Israel Syrian Mexico South China USA Africa Country Fig.2 Top 10 of Pomelo Production(2005-2007) The top 10 pomelo production countries (2005-2007) Source: The data come from http://www.fao.org) 350 290. 9 ) 300 T 0 234 0 250 0 1 × 200 ( 154. 3 t r o 150 p x 100 67. 6 E 42. 6 39. 9 53. 5 50 17. 9 23. 7 19. 2 0 el ca d s a m A a a y a i n ru in iu S li in e r fr la p h g ra t rk Is o y C l U t n u -A e s e T S H C B u rg A A Country The top 10 pomelo export countries(2006) Source: The data come from http://www.fao.org) ) T 200 170. 9 0 0 123. 6 0 150 1 × 84. 3 ( 100 t 63. 9 r 47. 7 54. 4 50 56. 5 o 27. 5 29. 5 p 50 m I 0 a . y e a d d K d n ia n m c i n a . an a al pa iu n ss a n U l m tr a lg ra u l a o r s J e F R o C H e u B P G A Country The top 10 pomelo import countries (2006) Source: The data come from http://www.fao.org) China is the country of the largest acreage of pomelo production, and its output rank second in the world, just next to USA There is a very long history (more than 3000 years) of pomelo cultivation. -
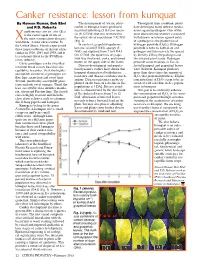
Canker Resistance: Lesson from Kumquat by Naveen Kumar, Bob Ebel the Development of Asiatic Citrus Throughout Their Evolution, Plants and P.D
Canker resistance: lesson from kumquat By Naveen Kumar, Bob Ebel The development of Asiatic citrus Throughout their evolution, plants and P.D. Roberts canker in kumquat leaves produced have developed many defense mecha- anthomonas citri pv. citri (Xcc) localized yellowing (5 DAI) or necro- nisms against pathogens. One of the is the causal agent of one of sis (9-12 DAI) that was restricted to most characteristic features associated the most serious citrus diseases the actual site of inoculation 7-12 DAI with disease resistance against entry X (Fig. 2). of a pathogen is the production of worldwide, Asiatic citrus canker. In the United States, Florida experienced In contrast, grapefruit epidermis hydrogen peroxide (H2O2). Hydrogen three major outbreaks of Asiatic citrus became raised (5 DAI), spongy (5 peroxide is toxic to both plant and canker in 1910, 1984 and 1995, and it DAI) and ruptured from 7 to 8 DAI. pathogen and thus restricts the spread is a constant threat to the $9 billion On 12 DAI, the epidermis of grape- by directly killing the pathogen and citrus industry. fruit was thickened, corky, and turned the infected plant tissue. Hydrogen Citrus genotypes can be classified brown on the upper side of the leaves. peroxide concentrations in Xcc-in- into four broad classes based on sus- Disease development and popula- fected kumquat and grapefruit leaves ceptibility to canker. First, the highly- tion dynamics studies have shown that were different. Kumquat produces susceptible commercial genotypes are kumquat demonstrated both disease more than three times the amount of Key lime, grapefruit and sweet lime. -

Chapter 1 Definitions and Classifications for Fruit and Vegetables
Chapter 1 Definitions and classifications for fruit and vegetables In the broadest sense, the botani- Botanical and culinary cal term vegetable refers to any plant, definitions edible or not, including trees, bushes, vines and vascular plants, and Botanical definitions distinguishes plant material from ani- Broadly, the botanical term fruit refers mal material and from inorganic to the mature ovary of a plant, matter. There are two slightly different including its seeds, covering and botanical definitions for the term any closely connected tissue, without vegetable as it relates to food. any consideration of whether these According to one, a vegetable is a are edible. As related to food, the plant cultivated for its edible part(s); IT botanical term fruit refers to the edible M according to the other, a vegetable is part of a plant that consists of the the edible part(s) of a plant, such as seeds and surrounding tissues. This the stems and stalk (celery), root includes fleshy fruits (such as blue- (carrot), tuber (potato), bulb (onion), berries, cantaloupe, poach, pumpkin, leaves (spinach, lettuce), flower (globe tomato) and dry fruits, where the artichoke), fruit (apple, cucumber, ripened ovary wall becomes papery, pumpkin, strawberries, tomato) or leathery, or woody as with cereal seeds (beans, peas). The latter grains, pulses (mature beans and definition includes fruits as a subset of peas) and nuts. vegetables. Definition of fruit and vegetables applicable in epidemiological studies, Fruit and vegetables Edible plant foods excluding