Carbon Allocation Strategies for Reproduction and Growth in Spring Ephemeral Plants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

HELLEBORUS NIGER (Hell. Nig.) Botanical Name : Helleborus Niger
HELLEBORUS NIGER (Hell. nig.) Botanical name : Helleborus niger Linn. Family: Ranunculaceae Synonyms : Elleborum nigrum, Helleborus grandiflorus Salisb., Veratrum nigrum Salisb. Common names : Hindi: Khorasani kutki; English: Black hellebore, Christmas rose; French: Ellebore noir; German: Sohwarze Uieswurzel. Description : A perennial, having brownish-black, knotted, brittle, fleshy rhizome, 2.5 to 7.5 cm long, 6 to 12 mm thick. Leaves on long stalks, which spring directly from the root. Stalks are cylindrical, tapering, smooth, shinning and pale green, mottled with red. Leaves pedate, deeply divided into several nearly separate lobes, coarsely seriate in the upper part, dark green above, paler beneath. Flowers on a scape shorter than petiole, at first pinkish-white, becoming green. Macroscopical : The drug occurs in irregularly branched, blackish pieces from 3.0 to 6.0 cm in length and from 5 to 8 mm in diameter. The branches show encircling leaf scars and the remains of the aerial stem or buds. Microscopical : Transverse sections of the rhizome shows considerable variations, 4 to 12 or more vascular bundles often of widely different shapes. Habitat : Found in alpine regions. History and authority : Introduced by Hahnemann in 1805. Allen’s Encyclop. Mat. Med. Vol. IV, 547. Part used : Rhizome. Moisture content of fresh rhizome 200 ml per 100 g solids. Preparation : (a) Mother Tincture φ Drug strength 1/10 Helleborus Niger in coarse powder 100 g Purified Water 400 ml Strong Alcohol 635 ml to make one thousand millilitres of the Mother Tincture. (b) Potencies: 2x contain one part tincture, three parts purified water and six parts Strong Alcohol. 3x and higher with Dispensing Alcohol. -

Big Bluestem
. Native Plants Appendix Saint Paul Parks and Recreation big bluestem Scientific Name: Andropogon geradii Description: ▫Perennial grass growing to a height of 3 to 10 feet. ▫Stem: stem base turns a blue-purple color as it matures ▫Root structure: deep roots; sends out rhizomes Flowers and seed heads: Flowers are spike-lets born in pairs. Three spike-like projections (looks like a turkey foot) form the seed head. Habitat: native to Minnesota and much of the tall grass prairies of the Great Plains in North America Planting Recommendations: prefers full sun, moist to slightly dry conditions, and fertile-loam or clay loam soil Fun Fact Big bluestem is also used as forage for livestock. Native Plants Appendix Saint Paul Parks and Recreation black-eyed susan Scientific Name: Rudbeckia hirta Description: ▫Annual or biennial herbaceous plant, 1 to 3 feet tall ▫Leaves: spirally arranged, entire to deeply lobed; covered in bristly hairs ▫Root system: central taproot and no rhizomes; reproduces entirely by seed ▫Flowers: the flower has dark brown disc florets and yellow or orange ray florets in a daisy-like shape. Habitat: native to United States Planting Recommendations: plant in full sun; prefers slightly moist to moderately dry soil conditions Best Display: has flowers present from June to August Common Problems: aphids and whiteflies; powdery mildew fungi Fun Facts It is also called a cone shaped head because when the flower head opens the ray florets have a tendency to point out and down. This plant is often used in prairie restoration and recovers moderately well from fires. Native Plants Appendix Saint Paul Parks and Recreation black raspberry Scientific Name: Rubus occidentalis Description: ▫Perennial deciduous shrub ▫Leaves: pinnate with five leaflets making up one leaf and three leaflets on stems with flowering branchlets. -

Helleborus Niger Helleborus Niger, Commonly Called Christmas Rose Or Black Hellebore, Is an Evergreen Perennial Flowering Plant in the Buttercup Family, Ranunculaceae
Helleborus niger Helleborus niger, commonly called Christmas rose or black hellebore, is an evergreen perennial flowering plant in the buttercup family, Ranunculaceae. It is poisonous. Although the flowers resemble wild roses (and despite its common name), Christmas rose does not belong to the rose family (Rosaceae). Taxonomy The black hellebore was described by Carl Linnaeus in volume one of his Species Plantarum in 1753.The Latin specific name niger (black) may refer to the colour of the roots. There are two subspecies: H. niger niger and H. niger macranthus, which has larger flowers (up to 3.75 in/9 cm across). In the wild, H. niger niger is generally found in mountainous areas in Switzerland, southern Germany, Austria, Slovenia, Croatia and northern Italy. Helleborus niger macranthus is found only in northern Italy and possibly adjoining parts of Slovenia. Description Helleborus niger is an evergreen plant with dark leathery pedate leaves carried on stems 9–12 in (23–30 cm) tall. The large flat flowers, borne on short stems from midwinter to early spring, are generally white, but occasionally purple or pink. The tips of the petals may be flushed pink or green, and there is a prominent central boss of yellow. Horticulture The plant is a traditional cottage garden favourite because it flowers in the depths of winter. Large- flowered cultivars are available, as are pink-flowered and double-floweredselections. It can be difficult to grow well; acid soil is unsuitable, as are poor, dry conditions and full sun. Moist, humus-rich, alkaline soil in dappled shade is preferable. Leaf-mould can be dug in to improve heavy clay or light sandy soils; lime can be added to 'sweeten' acid soils. -

In Vitro Propagation of Rosa 'Konstancin'
FOLIA HORTICULTURAE Folia Hort. 30(2), 2018, 259-267 Published by the Polish Society DOI: 10.2478/fhort-2018-0022 for Horticultural Science since 1989 ORIGINAL ARTICLE Open access www.foliahort.ogr.ur.krakow.pl In vitro propagation of Rosa ‘Konstancin’ (R. rugosa × R. beggeriana), a plant with high nutritional and pro-health value Agnieszka Wojtania*, Bożena Matysiak Department of Applied Biology Research Institute of Horticulture Konstytucji 3 Maja 1/3, 96-100 Skierniewice, Poland ABSTRACT The aim of the study was to develop an efficient micropropagation system forRosa ‘Konstancin’, an interspecific hybrid between R. rugosa and R. beggeriana, whose fruits have high pro-health value. Shoot cultures were initiated from shoot buds collected in May and August from 15-year-old field-grownRosa ‘Konstancin’ shrubs. The effect and interaction of different concentrations of phytohormones, sucrose and iron sources on in vitro initiation, multiplication and rooting of shoots were studied. The time of collecting explants from donor plants significantly affected the initiation of shoot culture ofRosa ‘Konstancin’. Considerably higher frequency of bud break (100%) was obtained in explants isolated in August as compared to those collected at the end of May (30%). All buds developed into single shoots after 2-4 weeks of growing on the basal Murashige and Skoog medium containing 2.2 µM BAP, 0.3 µM GA3 and 88 mM of sucrose. The highest multiplication rate (4.8 shoots/explant) in a 5-week period was obtained on MS medium containing 50% of nitrogen salts, 3.1 µM BAP, 0.9 µM GA3 and 58 mM sucrose. -
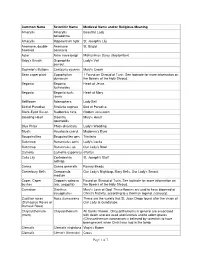
Of 7 Common Name Scientific Name Medieval Name And/Or Religious Meaning Amaryllis Amaryllis Belladonna Beautiful Lady
Common Name Scientific Name Medieval Name and/or Religious Meaning Amaryllis Amaryllis Beautiful Lady belladonna Amaryllis Hippeastrum hybr. St. Joseph's Lily Anemone, double- Anemone St. Brigid flowered coronaria Aster Aster nova-belgii Michaelmas Daisy (September) Baby's Breath Gypsophila Lady's Veil panicul. Bachelor's Buttons Centauria cyannis Mary's Crown Bean caper plant Zygophyllum ? Found on Shroud of Turin. See footnote for more information on dumosum the flowers of the Holy Shroud. Begonia Begonia Heart of Jesus fuchsioides Begonia Begonia fuch. Heart of Mary rosea Bellflower Adenophera Lady Bell Bird of Paradise Streliztia reginae Bird of Paradise Black-Eyed Susan Rudbeckia hirta Golden Jerusalem Bleeding Heart Dicentra Mary's Heart spectabilis Blue Phlox Phlox divaricata Lady's Wedding Bluets Houstonia caerul. Madonna's Eyes Bougainvillea Bougainvillea gen. Trinitaria Buttercup Ranunculus acris Lady's Locks Buttercup Ranunculus sp. Our Lady's Bowl Camelia Camellia (japonica) (Purity) Calla Lily Zantedeshia St. Joseph's Staff aethiop. Canna Canna generalis Rosary Beads Canterbury Bells Campanula Our Lady's Nightcap, Mary Bells, Our Lady's Smock medium Caper, Caper Capparis spinosa Found on Shroud of Turin. See footnote for more information on bushes (var. aegyptia) the flowers of the Holy Shroud. Carnation Dianthus Mary's Love of God. These flowers are said to have bloomed at caryophyllus Christ's Nativity, according to a German legend. (January) Castilian roses Rosa damascena These are the variety that St. Juan Diego found after the vision of (Damascus Roses or Our Lady at Guadalupe. Damask Rose) Chrysanthemum Chrysanthemum All Saints' Flower. Chrysanthemums in general are associated (mum) with death and are used and funerals and to adorn graves (Chrysanthemum coronarium is believed by scientists to have been present when Christ was laid in the tomb. -

Effect of Arbuscular Mycorrhizal Colonization on Ecological Functional Traits of Ephemerals in the Gurbantonggut Desert
SYMBIOSIS (2008) 46, 121-127 ©2008 Balaban, Philadelphia/Rehovot ISSN 0334-5114 Effect of arbuscular mycorrhizal colonization on ecological functional traits of ephemerals in the Gurbantonggut desert Y. Sun, X.L. Li, and G. Feng* College of Natural Resources and Environmental Sciences, China Agricultural University, Beijing I 00094, China, Tel. +86-10-62733885, Fax. +86-10-62731016, Email. [email protected] (Received August 14, 2007; Accepted February 7, 2008) Abstract The spring ephemerals are distinct and important flora in the Gurbantonggut desert, in central Asia and northwestern China. In order to understand the role of arbuscular mycorrhizal (AM) fungi on growth of ephemerals, a pot experiment was conducted in greenhouse conditions. Two desert ephemerals, Erodium oxyrrhynchum and Plantago minuta, were tested for their response to inoculation with two AM fungi, BEG 167 (Glomus mosseae) and BEG 141 (Glomus intraradices). The results showed that mycorrhizal colonization led to marked improvement in both the reproductive (timing of flowering and number of seeds) and vegetative (dry matter) phase of the two ephemeral plants. Dry weight per plant inoculated with AM fungi was 57 to 67 percent higher than the control in E. oxyrrhynchum and 8 to 11 times higher than the control in P. minuta. Anthesis was advanced by 14 to 17d in P minuta and 5 to 7d in E. oxyrrhynchum, respectively, when both plants were inoculated with AM fungi. Colonization of mycorrhizal fungi significantly increased the total number of seeds or fruits per plant. Water use efficiency and photosynthetic rates were significantly higher in inoculated E. oxyrrhynchum plants than those of non-inoculated plants. -

Late Canopy Closure Delays Senescence and Promotes Growth of the Spring Ephemeral Wild Leek ( Allium Tricoccum)
Botany Late canopy closure delays senescence and promotes growth of the spring ephemeral wild leek ( Allium tricoccum). Journal: Botany Manuscript ID cjb-2016-0317.R1 Manuscript Type: Article Date Submitted by the Author: 04-Feb-2017 Complete List of Authors: Dion, Pierre-Paul; Universite Laval, Phytologie Bussières, DraftJulie; Universite Laval, Biologie Lapointe, Line; Université Laval, Biologie Keyword: <i>Allium tricoccum</i>, Tree canopy, Light, Phenology, Spring ephemeral https://mc06.manuscriptcentral.com/botany-pubs Page 1 of 33 Botany Late canopy closure delays senescence and promotes growth of the spring ephemeral wild leek (Allium tricoccum ). Pierre-Paul DION 1, Julie BUSSIÈRES & Line LAPOINTE Centre for Forest Research and Department of Biology, Laval University, Québec, Québec, Canada, G1V 0A6. Pierre-Paul Dion: [email protected] Julie Bussières: [email protected] Line Lapointe: [email protected] Corresponding author: Pierre-Paul Dion,Draft Department of Plant Science, Laval University, Québec, Québec, Canada, G1V 0A6. Email: [email protected] 1 New affiliation: Department of Plant Science, Laval University, Québec, Québec, Canada, G1V 0A6. Email: [email protected] 1 https://mc06.manuscriptcentral.com/botany-pubs Botany Page 2 of 33 Abstract Spring ephemerals take advantage of the high light conditions in spring to accumulate carbon reserves through photosynthesis before tree leaves unfold. Recent work reports delayed leaf senescence under constant light availability in some spring ephemerals, such as wild leek ( Allium tricoccum ). This paper aims at establishing if tree canopy composition and phenology can influence the growth of spring ephemerals through changes in their phenology. -

Study of Germination Techniques for Helleborus Niger
A STUDY OF GERMINATION TECHINQUES FOR HELLEBORUS NIGER by SHARON J. LOCKHART B. S. Kansas State University 1982 A MASTER 1 S THESIS Submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Horticulture Kansas State University Manhattan, Kansas 1984 Approved by: ,Tf TABLE OF CONTENTS Lbl-I PAGE LIST OF TABLES i LIST OF FIGURES iii INTRODUCTION 1 LITERATURE REVIEW 7 MATERIALS AND METHODS 10 RESULTS 15 CONCLUSIONS 31 LITERATURE CITED 32 ACKNOWLEDGMENTS 35 APPENDIX 36 i List of Tables Table Page 1. Influence of stratification temperature, length of storage and GA3 treatment on Helleborus niger seed germination at 16°C 18 2. Effects of length of storage and temperature on germination of Hel leborus niger 19 3. Influence of stratification temperature, length of storage and KNO3 treatment on He 1 leborus niger seed germination 23 4. Storage period and temperature affects on He 1 1 eborus niger germination 160 and 250 days po s t- s t o rage . 24 5. Influence of stratification temperature, length of storage and chemical treatment on Helleborus niger seed germination 28 6. Comparison of constant and diurnal germination temperature and storage period on He 1 1 ebo rus niger germination 29 7. Effects of stratification temperature and length of storage on Helleborus niger seed germination 30 , 1 Introduct ion Helleborus niger , Christmas rose is a herbaceous perennial native to cool, moist woodland areas of Central and Southern Europe and the Alps. Hellebores, members of the Ranun cu 1 ac ea e are a favorite perennial garden selection and are forced as a cutf lower crop for the Christmas season (3,12,19,20,27). -

Source–Sink Imbalance Increases with Growth Temperature in the Spring Geophyte Erythronium Americanum
Journal of Experimental Botany, Vol. 62, No. 10, pp. 3467–3479, 2011 doi:10.1093/jxb/err020 Advance Access publication 18 February, 2011 This paper is available online free of all access charges (see http://jxb.oxfordjournals.org/open_access.html for further details) RESEARCH PAPER Source–sink imbalance increases with growth temperature in the spring geophyte Erythronium americanum Anthony Gandin1,3,*, Sylvain Gutjahr2, Pierre Dizengremel3 and Line Lapointe1 1 De´ partement de biologie et Centre d’e´ tude de la foreˆ t, Universite´ Laval, Que´ bec (QC), Canada G1V 0A6 2 CIRAD, UPR A˜ IVA, F-34398 Montpellier cedex 5, France 3 Faculte´ des Sciences et Techniques, UMR 1137 E´ cologie et e´ cophysiologie forestie` res, Nancy-Universite´ , BP 239, F-54506 Vandoeuvre, France * Present address and to whom correspondence should be sent: School of Biological Sciences, Washington State University, Pullman, WA 99164-4236, USA. E-mail: [email protected] Received 30 October 2010; Revised 11 January 2011; Accepted 17 January 2011 Abstract Spring geophytes produce larger storage organs and present delayed leaf senescence under lower growth temperature. Bulb and leaf carbon metabolism were investigated in Erythronium americanum to identify some of the mechanisms that permit this improved growth at low temperature. Plants were grown under three day/night temperature regimes: 18/14 °C, 12/8 °C, and 8/6 °C. Starch accumulated more slowly in the bulb at lower temperatures probably due to the combination of lower net photosynthetic rate and activation of a ‘futile cycle’ of sucrose synthesis and degradation. Furthermore, bulb cell maturation was delayed at lower temperatures, potentially due to the delayed activation of sucrose synthase leading to a greater sink capacity. -

Master Plant List
MASTER PLANT LIST 5 N 9 7 8 6 Glasshouse 4 Green Roof 1 2 3 7 MASTER PLANT LIST PAGE 1 TREES 4 Acer griseum PAPERBARK MAPLE 2 3 Acer palmatum ‘Atropurpureum Dissectum’ RED WEEPING CUT-LEAF JAPANESE MAPLE 3 4 5 6 7 Acer palmatum ‘Sango Kaku’ CORAL BARK JAPANESE MAPLE 7 Chamaecyparis nootkatensis ‘Pendula’ WEEPING NOOTKA CYPRESS 7 Chamaecyparis obtusa ‘Gracilis’ SLENDER HINOKI CYPRESS 1 6 Cornus rutgersensis ‘Celestial’ CELESTIAL DOGWOOD 3 6 Davidia involucrata ‘Sonoma’ SONOMA DOVE TREE 4 Gleditsia triacanthos inermis ‘Shademaster’ SHADEMASTER HONEY LOCUST 7 Magnolia grandiflora ‘Teddy Bear’ TEDDY BEAR MAGNOLIA 7 Magnolia grandiflora ‘Bracken’s Brown Beauty’ BRAKEN’S BROWN BEAUTY MAGNOLIA 3 Picea pungens ‘Iseli Fastigiate’ ISELI FASTIGIATE SPRUCE 3 7 Sciadopitys verticillata ‘Wintergreen’ WINTERGREEN UMBRELLA PINE 2 3 Stewartia pseudocamellia JAPANESE STEWARTIA 7 Thuja plicata ‘Atrovirens’ WESTERN RED CEDAR SHRUBS 8 Arbutus compacta DWARF STRAWBERRY TREE 7 Aucuba japonica ‘Rozannie’ ROSANNIE AUCUBA 7 Berberis x gladwynensis ‘William Penn’ BARBERRY 5 Buxus microphylla ‘Wintergreen’ BOXWOOD 8 Callicarpa ‘Profusion’ BEAUTY BERRY 5 7 Camellia sasanqua ‘Yuletide’ YULETIDE CAMELLIA 3 Camellia sasanqua ‘Setsugekka’ SETSUGEKKA CAMELLIA 5 Chaenomeles ‘Dragon’s Blood’ QUINCE 5 Chaenomeles ‘Scarlet Storm’ QUINCE 5 Cornus sericea ‘Bud’s Yellow’ YELLOWTWIG DOGWOOD 1 Corylus avellana ‘Contorta’ HARRY LAUDER’S WALKING STICK 6 Cryptomeria japonica ‘Black Dragon’ BLACK DRAGON JAPANESE CEDAR 8 Cotoneaster dammeri BEARBERRY 2 Daphne genkwa LILAC DAPHNE 4 Dichroa febrifuga CHINESE QUININE 2 Edgeworthia chrysantha ‘Snow Cream’ RICE PAPER SHRUB 7 Fatshedera lizei TREE IVY 7 x Fatshedera lizei ‘Variegata’ VARIGATED TREE IVY 5 Fothergilla gardenii DWARF WITCH ALDER 5 Hamamelis japonica ‘Shibamichi Red’ JAPANESE WITCH HAZEL 2 4 Hydrangea macrophylla ssp. -

Plants Toxic to Horses
Plants Toxic to Horses Adam-and-Eve (Arum, Lord-and-Ladies, Wake Robin, Starch Root, Bobbins, Cuckoo Plant) | Scientific Names: Arum maculatum | Family: Araceae African Wonder Tree () | Scientific Names: Ricinus communis | Family: Alocasia (Elephant's Ear) | Scientific Names: Alocasia spp. | Family: Araceae Aloe () | Scientific Names: Aloe vera | Family: Liliaceae Alsike Clover () | Scientific Names: Trifolium hybridum | Family: Leguminosae Amaryllis (Many, including: Belladonna lily, Saint Joseph lily, Cape Belladonna, Naked Lady) | Scientific Names: Amaryllis spp. | Family:Amaryllidaceae Ambrosia Mexicana (Jerusalem Oak, Feather Geranium) | Scientific Names: Chenopodium botrys | Family: Chenopodiaceae American Bittersweet (Bittersweet, Waxwork, Shrubby Bittersweet, False Bittersweet, Climbing Bittersweet) | Scientific Names: Celastrus scandens| Family: Celastraceae American Holly (English Holly, European Holly, Oregon Holly, Inkberry, Winterberry) | Scientific Names: Ilex opaca | Family: Aquifoliaceae American Mandrake (Mayapple, Indian Apple Root, Umbrella Leaf, Wild Lemon, Hog Apple, Duck's Foot, Raccoonberry) | Scientific Names:Podophyllum peltatum | Family: Berberidaceae American Yew (Canada Yew, Canadian Yew) | Scientific Names: Taxus canadensus | Family: Taxaceae Andromeda Japonica (Pieris, Lily-of-the-Valley Bush) | Scientific Names: Pieris japonica | Family: Ericaceae Angelica Tree (Hercules' Club, Devil's Walking Stick, Prickly Ash, Prickly Elder) | Scientific Names: Aralia spinosa | Family: Araliaceae Apple (Includes crabapples) -

POISONOUS PLANTS for PEOPLE Commonly Know As: Scientific Name: Poisonous Parts: Aconite;Monkshood;Wolfbane Aconitum All Parts Alstromeria Alstroemeria Spp
Fred Hall-CEA Agriculture/NR 600 Scott Street, Suite 200 Wichita Falls, Texas 76301 POISONOUS PLANTS FOR PEOPLE Commonly know as: Scientific Name: Poisonous Parts: Aconite;Monkshood;Wolfbane Aconitum All parts Alstromeria Alstroemeria spp. Leaves, stems (dermatitis) Angel Trumpet Brugmanisa All parts Apple Malus spp. Seeds in quantity Azalea; Rhododendron Rhododendron spp. All parts Belladonna Atropa belladonna All parts Bittersweet, Climbing or American Celastrus scandens Fruits Bittersweet; Nightshade Solanum dulcamara Stems, leave, berries Bleeding Heart Dicentra spp. All parts Bluebonnets Lupinus spp. All parts Bracken Fern Pteridium aquilinum All parts Calla Lily Calla spp. All parts Carnation Dianthus caryophyllus All parts Carolina Jessamine, Yellow Glesemium sepmervirens All parts Castor Bean Ricinus communis Foliage, seeds China Berry Melia azedarach Berries Caladium Caladium spp. Leaves, roots Corn poppy:Red poppy;Field poppy Papaver rhoeas All parts Crocus, Autumn; Meadow Saffron Colchicum autumnal All parts Cyclamen Cyclamen spp. Roots Delphinium; Larkspur Delphinium spp. All parts Dumb cane Dieffenbachia spp. All parts Elephant Ear Philodendron spp. All parts Flame Lily Gloriosa superba All parts Four-O Clock Mirabilis spp. Roots, seeds Foxglove; Digitalis Digitalis purpurea All parts Colden Chain Tree Laburnum anagyroides Fruits Hellebore, White Veratrum album All parts Hellebore; Christmas Rose Helleborus niger All parts Henbane, Black Hyoscyamus niger All parts Holly, English Hex aquifolium Berries Commonly know as: Scientific Name: Poisonous Parts: Hyacinth Hyacinths spp. All parts Iris Iris spp. Underground stems, leaves Ivy, English Hedera helix Leaves, berries Jerusalem Cherry Solanum pseudo capsicum Leaves, berries Jimsonweed, Thorn-Apple, Datura Datura stramonium & spp. All parts Lantana Lantana camara Young berries Laurel Cherry, Spp.