Temperature, Physiology, and the Ecology of Reptiles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Do Worm Lizards Occur in Nebraska? Louis A
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Herpetology Papers in the Biological Sciences 1993 Do Worm Lizards Occur in Nebraska? Louis A. Somma Florida State Collection of Arthropods, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/biosciherpetology Part of the Biodiversity Commons, and the Population Biology Commons Somma, Louis A., "Do Worm Lizards Occur in Nebraska?" (1993). Papers in Herpetology. 11. http://digitalcommons.unl.edu/biosciherpetology/11 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Papers in Herpetology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. @ o /' number , ,... :S:' .' ,. '. 1'1'13 Do Mono Li ••rel,. Occur ill 1!I! ..br .... l< .. ? by Louis A. Somma Department of- Zoology University of Florida Gainesville, FL 32611 Amphisbaenids, or worm lizards, are a small enigmatic suborder of reptiles (containing 4 families; ca. 140 species) within the order Squamata, which include~ the more speciose lizards and snakes (Gans 1986). The name amphisbaenia is derived from the mythical Amphisbaena (Topsell 1608; Aldrovandi 1640), a two-headed beast (one head at each end), whose fantastical description may have been based, in part, upon actual observations of living worm lizards (Druce 1910). While most are limbless and worm-like in appearance, members of the family Bipedidae (containing the single genus Sipes) have two forelimbs located close to the head. This trait, and the lack of well-developed eyes, makes them look like two-legged worms. -

Effect of Ethanol on Thermoregulation in the Goldfish, Carassius Auratus
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 1986 Effect of ethanol on thermoregulation in the goldfish, Carassius auratus Candace Sharon O'Connor Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Biology Commons, and the Physiology Commons Let us know how access to this document benefits ou.y Recommended Citation O'Connor, Candace Sharon, "Effect of ethanol on thermoregulation in the goldfish, Carassius auratus" (1986). Dissertations and Theses. Paper 3703. https://doi.org/10.15760/etd.5587 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. AN ABSTRACT OF THE THESIS of Candace Sharon O'Connor for the Master of Science in Biology presented May 16, 1986. Title: Effect of Ethanol on Thermoregulation in the Goldfish, Carassius auratus. APPROVED BY MEMBERS OF THE TIIBSIS COMMITTEE: Leonard Simpson In an attempt to elucidate the mechanism by which ethanol affects vertebrate thermoregulation, the effect of ethanol on temperature selection was studied in the goldfish, Carassius auratus. Ethanol was administered to 10 to 15 g fish by mixing it in the water of a temperature gradient. The dose response curve was very steep between 0.5% (v/v) ethanol (no response) and 0.7% (significant lowering of selected temperature in treated fish). Fish were exposed to concentrations of ethanol as high as 1.7%, at which concentration most experimental fish lost their ability to swim upright in the water. -

Ostrich Production Systems Part I: a Review
11111111111,- 1SSN 0254-6019 Ostrich production systems Food and Agriculture Organization of 111160mmi the United Natiorp str. ro ucti s ct1rns Part A review by Dr M.M. ,,hanawany International Consultant Part II Case studies by Dr John Dingle FAO Visiting Scientist Food and , Agriculture Organization of the ' United , Nations Ot,i1 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-21 ISBN 92-5-104300-0 Reproduction of this publication for educational or other non-commercial purposes is authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Information Division, Food and Agriculture Organization of the United Nations, Viale dells Terme di Caracalla, 00100 Rome, Italy. C) FAO 1999 Contents PART I - PRODUCTION SYSTEMS INTRODUCTION Chapter 1 ORIGIN AND EVOLUTION OF THE OSTRICH 5 Classification of the ostrich in the animal kingdom 5 Geographical distribution of ratites 8 Ostrich subspecies 10 The North -

Origin of Tropical American Burrowing Reptiles by Transatlantic Rafting
Biol. Lett. in conjunction with head movements to widen their doi:10.1098/rsbl.2007.0531 burrows (Gans 1978). Published online Amphisbaenians (approx. 165 species) provide an Phylogeny ideal subject for biogeographic analysis because they are limbless (small front limbs are present in three species) and fossorial, presumably limiting dispersal, Origin of tropical American yet they are widely distributed on both sides of the Atlantic Ocean (Kearney 2003). Three of the five burrowing reptiles by extant families have restricted geographical ranges and contain only a single genus: the Rhineuridae (genus transatlantic rafting Rhineura, one species, Florida); the Bipedidae (genus Nicolas Vidal1,2,*, Anna Azvolinsky2, Bipes, three species, Baja California and mainland Corinne Cruaud3 and S. Blair Hedges2 Mexico); and the Blanidae (genus Blanus, four species, Mediterranean region; Kearney & Stuart 2004). 1De´partement Syste´matique et Evolution, UMR 7138, Syste´matique, Evolution, Adaptation, Case Postale 26, Muse´um National d’Histoire Species in the Trogonophidae (four genera and six Naturelle, 57 rue Cuvier, 75231 Paris Cedex 05, France species) are sand specialists found in the Middle East, 2Department of Biology, 208 Mueller Laboratory, Pennsylvania State North Africa and the island of Socotra, while the University, University Park, PA 16802-5301, USA largest and most diverse family, the Amphisbaenidae 3Centre national de se´quenc¸age, Genoscope, 2 rue Gaston-Cre´mieux, CP5706, 91057 Evry Cedex, France (approx. 150 species), is found on both sides of the *Author and address for correspondence: De´partment Syste´matique et Atlantic, in sub-Saharan Africa, South America and Evolution, UMR 7138, Syste´matique, Evolution, Adoptation, Case the Caribbean (Kearney & Stuart 2004). -

Effect of Body Temperature on the Pattern of Spontaneous Breathing in Extremely Low Birth Weight Infants Supported by Proportional Assist Ventilation
0031-3998/03/5403-0332 PEDIATRIC RESEARCH Vol. 54, No. 3, 2003 Copyright © 2003 International Pediatric Research Foundation, Inc. Printed in U.S.A. Effect of Body Temperature on the Pattern of Spontaneous Breathing in Extremely Low Birth Weight Infants Supported by Proportional Assist Ventilation ESTHER RIEGER-FACKELDEY, SUSANNE SCHALLER-BALS, AND ANDREAS SCHULZE Department of Obstetrics & Gynecology–Grosshadern, Division of Neonatology, Ludwig Maximilian University of Munich, D-81377 Munich, Germany ABSTRACT The optimum body temperature for infants Ͻ1000 g is un- 0.001) as a result of a difference in RR (8%; p Ͻ 0.001). The Ϯ known. We investigated body temperature effects on spontane- infants maintained their blood CO2 levels and Vt (5.25 0.6 ous breathing using proportional assist ventilation (PAV), be- versus 5.19 Ϯ 0.6 mL/kg). Incidence and duration of respiratory cause this mode supports spontaneous breathing such that all pauses were not different between conditions. Extremely imma- breathing pattern variables remain controlled by the infant. ture infants who are supported by PAV modify their spontaneous Minute volume (MV), respiratory rate (RR), tidal volume (Vt), breathing in response to changes in thermal environment such incidence and duration of respiratory pauses, arterial oxygen that PCO2 levels are appropriately maintained early in postnatal Ͻ desaturations 85%, and arterial PCO2 levels will remain unaf- life. This response pattern occurred consistently and is currently fected by targeting core body temperature to 36.1–36.5°C (low of uncertain clinical significance. (Pediatr Res 54: 332–336, normal range) versus 37.7–37.9°C (upper normal). -

Evolution of Limblessness
Evolution of Limblessness Evolution of Limblessness Early on in life, many people learn that lizards have four limbs whereas snakes have none. This dichotomy not only is inaccurate but also hides an exciting story of repeated evolution that is only now beginning to be understood. In fact, snakes represent only one of many natural evolutionary experiments in lizard limblessness. A similar story is also played out, though to a much smaller extent, in amphibians. The repeated evolution of snakelike tetrapods is one of the most striking examples of parallel evolution in animals. This entry discusses the evolution of limblessness in both reptiles and amphibians, with an emphasis on the living reptiles. Reptiles Based on current evidence (Wiens, Brandley, and Reeder 2006), an elongate, limb-reduced, snakelike morphology has evolved at least twenty-five times in squamates (the group containing lizards and snakes), with snakes representing only one such origin. These origins are scattered across the evolutionary tree of squamates, but they seem especially frequent in certain families. In particular, the skinks (Scincidae) contain at least half of all known origins of snakelike squamates. But many more origins within the skink family will likely be revealed as the branches of their evolutionary tree are fully resolved, given that many genera contain a range of body forms (from fully limbed to limbless) and may include multiple origins of snakelike morphology as yet unknown. These multiple origins of snakelike morphology are superficially similar in having reduced limbs and an elongate body form, but many are surprisingly different in their ecology and morphology. This multitude of snakelike lineages can be divided into two ecomorphs (a are surprisingly different in their ecology and morphology. -

Molecular Phylogenetics and Evolution 55 (2010) 153–167
Molecular Phylogenetics and Evolution 55 (2010) 153–167 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Conservation phylogenetics of helodermatid lizards using multiple molecular markers and a supertree approach Michael E. Douglas a,*, Marlis R. Douglas a, Gordon W. Schuett b, Daniel D. Beck c, Brian K. Sullivan d a Illinois Natural History Survey, Institute for Natural Resource Sustainability, University of Illinois, Champaign, IL 61820, USA b Department of Biology and Center for Behavioral Neuroscience, Georgia State University, Atlanta, GA 30303-3088, USA c Department of Biological Sciences, Central Washington University, Ellensburg, WA 98926, USA d Division of Mathematics & Natural Sciences, Arizona State University, Phoenix, AZ 85069, USA article info abstract Article history: We analyzed both mitochondrial (MT-) and nuclear (N) DNAs in a conservation phylogenetic framework to Received 30 June 2009 examine deep and shallow histories of the Beaded Lizard (Heloderma horridum) and Gila Monster (H. Revised 6 December 2009 suspectum) throughout their geographic ranges in North and Central America. Both MTDNA and intron Accepted 7 December 2009 markers clearly partitioned each species. One intron and MTDNA further subdivided H. horridum into its Available online 16 December 2009 four recognized subspecies (H. n. alvarezi, charlesbogerti, exasperatum, and horridum). However, the two subspecies of H. suspectum (H. s. suspectum and H. s. cinctum) were undefined. A supertree approach sus- Keywords: tained these relationships. Overall, the Helodermatidae is reaffirmed as an ancient and conserved group. Anguimorpha Its most recent common ancestor (MRCA) was Lower Eocene [35.4 million years ago (mya)], with a 25 ATPase Enolase my period of stasis before the MRCA of H. -

Investigating the Thermal Biology of Round Gobies by Justin Mark
Behavioural Thermoregulation and Escape Behaviour: Investigating the Thermal Biology of Round Gobies By Justin Mark Bridgeman Bachelor of Science, 2014, Brock University Submitted in partial fulfillment of the requirements for the degree of Master of Science Brock University, Faculty of Mathematics and Science Department of Biological Sciences St. Catharines, Ontario © 2019 Abstract The invasive round goby (Neogobius melanostomus) has successfully colonized all of the Great Lakes since its discovery in the region in 1991, yet little is known about its thermal biology. The focus of this thesis was to examine the effect of acclimation to unseasonably warm temperatures on round goby behavioural thermoregulation, as well as behavioural and physiological performance during escapes with warm acute temperatures. Juvenile gobies were acclimated to either 21°C or 24°C for each set of experiments. I first examined goby thermal preference in a shuttlebox through their ability to escape from unfavourable temperatures. I found that escape temperatures were plastic following acclimation to a rise in 3°C rise in temperature (+3°C) and associated positively with acclimation temperature, even though gobies showed slightly lower-than-expected average escape temperatures in each acclimation treatment. Interestingly, acclimation to +3°C leads to lower exploratory behaviour in warm waters and lower overall activity levels during behavioural thermoregulation. In risky situations involving threat of predation, exploratory behaviour is often linked to boldness. Next I investigated exploratory swimming through two behavioural traits: ability to voluntarily enter a tunnel and subsequent swimming activity while being chased in a detour task. Detour tasks require a fish to swim down a narrow space and then detour to the left or right as they approach a barrier. -

Mammalogy Lecture 17 – Thermoregulation/Water Balance I
Mammalogy Lecture 17 – Thermoregulation/Water Balance I. Introduction. Obviously, mammals are endotherms; they regulate body temperature via metabolic processes by burning energy. For all endotherms, there is a Thermal Neutral Zone When TA is low, energy is expended to keep warm. When TA is high, energy is expended to keep cool But for every endothermic species, there is a thermal neutral zone, the range of ambient temperatures across which there’s no higher cost of homeothermy. TLC - highest temperature at which an endotherm expends energy to stay warm TUC - lowest temperature at which an endotherm expends energy to stay cool Obviously, when ambient temperatures are either below TLC or above TUC, there is a metabolic cost to homeothermy (maintaining a constant body temperature). II. Adaptations for Cold – Temperatures in Zone A A. Large Size - B. High Basal Metabolic Rate - Cold adapted species have a higher than expected basal metabolic rate. For example, Red foxes, Vulpes vulpes, have a BMR that’s nearly twice as high as similar sized canids in warmer regions. C. Insulation - Pelage - forms a barrier of warm air next to the surface of the animal. Blubber - subcutaneous fat is commonly used as an insulating mechanism in marine mammals. D. Regional Heterothermy Extremities may be allowed to cool, sometimes to very low temperatures. This is accomplished by vasoconstriction. Urocitellus paryii - toe pads may be 2 o - 5 o C Ondatra zibethicus – extremities are allowed to cool to water temperature E. Systemic Heterothermy – Adaptive Hypothermia Characterized by: - Decreased heart rate - Vasoconstriction - severe reduction of blood flow to the extremities - Decreased breathing rate - Suppression of shivering - Decreased oxygen consumption (decreased metabolic rate) - Decreased body temperature There is usually great energy savings associated with hypothermia. -

The Evolution of Endothermy and Its Diversity in Mammals and Birds Author(S): Gordon C
Division of Comparative Physiology and Biochemistry, Society for Integrative and Comparative Biology The Evolution of Endothermy and Its Diversity in Mammals and Birds Author(s): Gordon C. Grigg, Lyn A. Beard, and Michael L. Augee Source: Physiological and Biochemical Zoology, Vol. 77, No. 6, Sixth International Congress of Comparative Physiology and Biochemistry Symposium Papers: Evolution and Advantages of Endothermy (November/December 2004), pp. 982-997 Published by: The University of Chicago Press. Sponsored by the Division of Comparative Physiology and Biochemistry, Society for Integrative and Comparative Biology Stable URL: http://www.jstor.org/stable/10.1086/425188 . Accessed: 08/11/2015 23:11 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp . JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. The University of Chicago Press and Division of Comparative Physiology and Biochemistry, Society for Integrative and Comparative Biology are collaborating with JSTOR to digitize, preserve and extend access to Physiological and Biochemical Zoology. http://www.jstor.org This content downloaded from 23.235.32.0 on Sun, 8 Nov 2015 23:11:10 PM All use subject to JSTOR Terms and Conditions 982 The Evolution of Endothermy and Its Diversity in Mammals and Birds Gordon C. Grigg1 thermy, including the capacity for homeothermic endothermy Lyn A. -

Fauna of Australia 2A
FAUNA of AUSTRALIA 26. BIOGEOGRAPHY AND PHYLOGENY OF THE SQUAMATA Mark N. Hutchinson & Stephen C. Donnellan 26. BIOGEOGRAPHY AND PHYLOGENY OF THE SQUAMATA This review summarises the current hypotheses of the origin, antiquity and history of the order Squamata, the dominant living reptile group which comprises the lizards, snakes and worm-lizards. The primary concern here is with the broad relationships and origins of the major taxa rather than with local distributional or phylogenetic patterns within Australia. In our review of the phylogenetic hypotheses, where possible we refer principally to data sets that have been analysed by cladistic methods. Analyses based on anatomical morphological data sets are integrated with the results of karyotypic and biochemical data sets. A persistent theme of this chapter is that for most families there are few cladistically analysed morphological data, and karyotypic or biochemical data sets are limited or unavailable. Biogeographic study, especially historical biogeography, cannot proceed unless both phylogenetic data are available for the taxa and geological data are available for the physical environment. Again, the reader will find that geological data are very uncertain regarding the degree and timing of the isolation of the Australian continent from Asia and Antarctica. In most cases, therefore, conclusions should be regarded very cautiously. The number of squamate families in Australia is low. Five of approximately fifteen lizard families and five or six of eleven snake families occur in the region; amphisbaenians are absent. Opinions vary concerning the actual number of families recognised in the Australian fauna, depending on whether the Pygopodidae are regarded as distinct from the Gekkonidae, and whether sea snakes, Hydrophiidae and Laticaudidae, are recognised as separate from the Elapidae. -
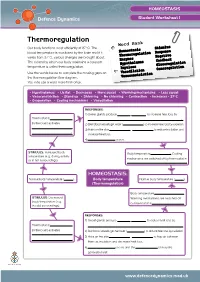
Thermoregulation Word Bank Our Body Functions Most Efficiently at 37°C
HOMEOSTASIS Student Worksheet 1 Thermoregulation Word Bank Our body functions most efficiently at 37°C. The Stimulus blood temperature is monitored by the brain and if it Homeostasis Response Thermoregulation varies from 37°C, various changes are brought about. Negative Enzymes The means by which our body maintains a constant feedback Hypothalamus Glucoregulation temperature is called thermoregulation. Impulses Osmoregulation Use the words below to complete the missing gaps on Vasodilation the thermoregulation flow diagram. Vasoconstriction You may use a word more than once. • Hypothalamus • Lie flat • Decreases • More sweat • Warming mechanisms • Less sweat • Vasoconstriction • Stand up • Shivering • No shivering • Contraction • Increases• 37ºC • Evaporation • Cooling mechanisms • Vasodilation RESPONSES: 1) Sweat glands produce more sweat to increase heat loss by Thermostat in . (in the brain) activates 2) Skin blood vessels get wider (vasodilatio n) to increase heat loss by radiation. 3) Hairs on the skin lie flat to reduce insulation and . increase heat loss. 4) No shivering occurs. STIMULUS: Increased body Body temperature decreases . Cooling temperature (e.g. during activity mechanisms are switched off by thermostat in or in hot surroundings). HOMEOSTASIS: Normal body temperature (37 ). Body temperature Normal body temperature (37 ). (Thermoregulation) Body temperature increas es. STIMULUS: Decreased Warming mechanisms are switched off body temperature (e.g. by thermostat in . in cold surroundings). RESPONSES: 1) Sweat glands produce more sweat to reduce heat loss by Thermostat in . (in the brain) activates 2) Skin blood vessels get narrower (vasodilatio n) to reduce heat loss by radiation. 3) Hairs on the skin lie flat to trap air between . them as insulation and decrease heat loss.