Gero Decher, Jean-Claude Voegel La Recherche, No
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Surface Polarization Effects in Confined Polyelectrolyte Solutions
Surface polarization effects in confined polyelectrolyte solutions Debarshee Bagchia , Trung Dac Nguyenb , and Monica Olvera de la Cruza,b,c,1 aDepartment of Materials Science and Engineering, Northwestern University, Evanston, IL 60208; bDepartment of Chemical and Biological Engineering, Northwestern University, Evanston, IL 60208; and cDepartment of Physics and Astronomy, Northwestern University, Evanston, IL 60208 Contributed by Monica Olvera de la Cruz, June 24, 2020 (sent for review April 21, 2020; reviewed by Rene Messina and Jian Qin) Understanding nanoscale interactions at the interface between ary conditions (18, 19). However, for many biological settings two media with different dielectric constants is crucial for con- as well as in supercapacitor applications, molecular electrolytes trolling many environmental and biological processes, and for confined by dielectric materials, such as graphene, are of interest. improving the efficiency of energy storage devices. In this Recent studies on dielectric confinement of polyelectrolyte by a contributed paper, we show that polarization effects due to spherical cavity showed that dielectric mismatch leads to unex- such dielectric mismatch remarkably influence the double-layer pected symmetry-breaking conformations, as the surface charge structure of a polyelectrolyte solution confined between two density increases (20). The focus of the present study is the col- charged surfaces. Surprisingly, the electrostatic potential across lective effects of spatial confinement by two parallel surfaces -
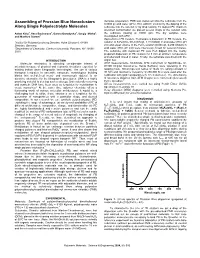
Assembling of Prussian Blue Nanoclusters Along Single
Assembling of Prussian Blue Nanoclusters Samples preparation. PMB was deposited onto the substrate from the 0.0005 g/l acid water (pH 2, HCl, Aldrich) solution by the dipping of the Along Single Polyelectrolyte Molecules substrate into the solution or by drop casting. To deposit PC chains in stretched conformation we placed several drops of the solution onto Anton Kiriy1, Vera Bocharova1, Ganna Gorodyska1, Sergiy Minko2, the substrate rotating at 10000 rpm. The dry samples were and Manfred Stamm1 investigated with AFM. Deposition of PB clusters. To prepare a dispersion of PB clusters, the 1 Institut für Polymerforschung Dresden, Hohe Strasse 6, 01069 solution of K4Fe(CN)6·3H20 (0.5 g/l, 1.18 mMol/l) in acid water (HCl, pH Dresden, Germany 2.0) and equal volume of the FeCl3 solution (0.048 g/l, 0.296 mMol/l) in 2Department of Chemistry, Clarkson University, Potsdam, NY 13699- acid water (HCl, pH 2.0) were intensively mixed for several minutes. 5810 The substrate with deposited PC was then dipped into the freshly prepared dispersion of PB clusters for 3 min at ambient temperature and afterward rinsed in water. Finally, the substrate was dried with the INTRODUCTION Argon flux. Molecular electronics is attracting considerable interest of AFM measurements. Multimode AFM instrument or NanoScope IV- scientists because of physical and economic limitations expected for D3100 (Digital Instruments, Santa Barbara) were operating in the existing bottom down lithographic technologies. The use of various tapping mode. Silicon tips with radius of 10-20 nm, spring constant of biological templates to assemble nanoscale nonbiological building 30 N/m and resonance frequency of 250-300 KHz were used after the blocks into well-defined meso- and macroscopic objects1 is nn calibration with gold nanoparticles (5 nm in diameter). -

Localized Enzyme-Assisted Self-Assembly in the Presence of Hyaluronic Acid for Hybrid Supramolecular Hydrogel Coating
polymers Communication Localized Enzyme-Assisted Self-Assembly in the Presence of Hyaluronic Acid for Hybrid Supramolecular Hydrogel Coating Jennifer Rodon Fores 1 , Alexis Bigo-Simon 1 ,Déborah Wagner 1, Mathilde Payrastre 1, Camille Damestoy 1, Lucille Blandin 1, Fouzia Boulmedais 1, Julien Kelber 1, Marc Schmutz 1 , Morgane Rabineau 2,3, Miryam Criado-Gonzalez 1,2,3,* , Pierre Schaaf 1,2,3,* and Loïc Jierry 1,* 1 Institut Charles Sadron (UPR22), Université de Strasbourg, CNRS, 23 rue du Loess, CEDEX 2, BP 84047, 67034 Strasbourg, France; [email protected] (J.R.F.); [email protected] (A.B.-S.); [email protected] (D.W.); [email protected] (M.P.); [email protected] (C.D.); [email protected] (L.B.); [email protected] (F.B.); [email protected] (J.K.); [email protected] (M.S.) 2 Institut National de la Santé et de la Recherche Médicale, INSERM Unité 1121, CRBS, 1 rue Eugène Boeckel, CEDEX, 67085 Strasbourg, France; [email protected] 3 Faculté de Chirurgie Dentaire, Université de Strasbourg, 8 rue Sainte Elisabeth, 67000 Strasbourg, France * Correspondence: [email protected] (M.C.-G.); [email protected] (P.S.); [email protected] (L.J.); Tel.: +91-25-8-74-97 (M.C.-G.); +33-3-68-85-33-87 (P.S.); +33-3-68-41-41-47(L.J.) Abstract: Hydrogel coating is highly suitable in biomaterial design. It provides biocompatibility and avoids protein adsorption leading to inflammation and rejection of implants. Moreover, hydrogels can be loaded with biologically active compounds. In this field, hyaluronic acid has been largely studied as an additional component since this polysaccharide is naturally present in extracellular matrix. -

Electrostatic Effects in Soft Matter and Biophysics NATO Science Series
Electrostatic Effects in Soft Matter and Biophysics NATO Science Series A Series presenting the results of scientific meetings supported under the NArO Science Programme. The Series is published by 10S Press, Amsterdam, and Kluwer Academic Publishers in conjunction with the NATO Scientific Affairs Division Sub-Series 1. Life and Behavioural Sciences 10S Press II. Mathematics, Physics and Chemistry Kluwer Academic Publishers III. Computer and Systems Science 10S Press IV. Earth and Environmental Sciences Kluwer Academic Publishers The NATO Science Series continues the series of books published formerly as the NATO ASI Series. The NATO Science Programme offers support for collaboration in civil science between scientists of countries of the Euro-Atlantic Partnership Council. The types of scientific meeting generally supported are "Advanced Study Institutes" and "Advanced Research Workshops", and the NATO Science Series collects together the results of these meetings. The meetings are co-organized bij scientists from NATO countries and scientists from NATO's Partner countries - countries of the CIS and Central and Eastern Europe. Advanced Study Institutes are high-Ievel tutorial courses offering in-depth study of latest advances in a field. Advanced Research Workshops are expert meetings aimed at critical assessment of a field, and identification of directions for future aetion. As a consequence of the restrueturing of the NATO Science programme in 1999, the NATO Science Series was re-organized to the four sub-series noted above. Please consult the following web sites for information on previous volumes published in the Series. http://www.nato.intlscience http://www.wkap.nl http://www.iospress.nl http://www.wtv-books.de/nato-pco.htm Series II: Mathematics, Physics and Chemistry - Voi. -

Gelation-Driven Component Selection in the Generation of Constitutional Dynamic Hydrogels Based on Guanine-Quartet Formation
Gelation-driven component selection in the generation of constitutional dynamic hydrogels based on guanine-quartet formation Nampally Sreenivasachary and Jean-Marie Lehn* Laboratoire de Chimie Supramole´culaire, Institut de Science et d’Inge´nierie Supramole´culaires (ISIS), Universite´Louis Pasteur, 8 Alle´e Gaspard Monge, BP 70028, 67083 Strasbourg Cedex, France Contributed by Jean-Marie Lehn, March 1, 2005 The guanosine hydrazide 1 yields a stable supramolecular hydrogel Such processes form the basis of the recently developed dynamic based on the formation of a guanine quartet (G-quartet) in pres- combinatorial chemistry (5, 6), in which molecular recognition ence of metal cations. The effect of various parameters (concen- events have been implemented toward the generation of optimal tration, nature of metal ion, and temperature) on the properties of binding agents toward artificial or biological (7) molecular recep- this gel has been studied. Proton NMR spectroscopy is shown to tors through a target-driven shift in the distribution of the library allow a molecular characterization of the gelation process. Hydra- constituents toward the best binder(s). Changes in library constit- zide 1 and its assemblies can be reversibly decorated by acylhy- uents may also be caused by redistribution of components induced drazone formation with various aldehydes, resulting in formation by metal ion binding (8, 9) or environmental factors such as of highly viscous dynamic hydrogels. When a mixture of aldehydes temperature and pH (N. Giuseppone and J.-M.L., unpublished is used, the dynamic system selects the aldehyde that leads to the data). most stable gel. Mixing hydrazides 1, 9 and aldehydes 6, 8 in 1:1:1:1 The amplification of a given constituent of a CDL under the ratio generated a constitutional dynamic library containing the pressure of a self-organization process, such as the formation of an four acylhydrazone derivatives A, B, C, and D. -

UCLA Electronic Theses and Dissertations
UCLA UCLA Electronic Theses and Dissertations Title A Fundamental Perspective on Polyelectrolyte Coagulants and Flocculants in Water Treatment Permalink https://escholarship.org/uc/item/5f30h7k4 Author Bhattacharya, Arkadeep Publication Date 2021 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA Los Angeles A Fundamental Perspective on Polyelectrolyte Coagulants and Flocculants in Water Treatment A thesis submitted in partial satisfaction of the requirements for the degree Master of Science in Chemical Engineering by Arkadeep Bhattacharya 2021 ABSTRACT OF THE THESIS A Fundamental Perspective on Polyelectrolyte Coagulants and Flocculants in Water Treatment by Arkadeep Bhattacharya Master of Science in Chemical Engineering University of California Los Angeles, 2021 Professor Samanvaya Srivastava, Chair Coagulation and flocculation are important phenomena which find widespread applications in water treatment. Polyelectrolytes are charged macromolecules which have found relevance in this domain due to their proven efficiency and effectiveness. The objective of the thesis would be to review and emphasize the fundamental mechanisms on which both natural and synthetic polyelectrolyte coagulants and flocculants operate. Advances in understanding phase characteristics and structure of aggregated polyelectrolyte complexes post interaction with charged impurities are discussed. These would help elucidate the correlation between salient polyelectrolyte properties -

Dispersed and Deposited Polyelectrolyte Complexes and Their Interactions to Chiral Compounds and Proteins
Dispersed and deposited polyelectrolyte complexes and their interactions to chiral compounds and proteins Dissertation zur Erlangung des akademischen Grades Doctor rerum naturalium (Dr. rer. nat.) vorgelegt der Fakultät Mathematik und Naturwissenschaften der Technischen Universität Dresden von M. Sc. Wuye Ouyang geboren am 01.05.1979 in Zhenjiang, V.R. China Gutachter : Prof. Dr. Brigitte Voit Prof. Dr. Thomas Wolff Prof. Dr. Klaus D. Jandt Eingereicht am : 10.10.2008 Tag der Verteidigung: 14.01.2009 ABBREVIATION AFM Atomic force microscopy ASC Ascorbic acid ATR-FTIR Attenuated Total Reflectance - Fourier Transform Infrared Spectroscopy CD Circular dichroism COAC Coacervate phase CSA Camphorsulfonic acid CSP Chiral stationary phases D2O Heavy water / Deuterium oxide DLS Dynamic light scattering DMF Dimethylformamide EtOH Ethanol GA Glutardialdehyde GLU Glutamic acid H2SO4 Sulfuric acid H2O2 Hydrogen peroxide HCl Hydrochloric acid HSA Human serum albumin IEP Isoelectric point IR Infrared IRE Internal reflection element LB Langmuir-Blodgett film LBL Layer by layer LYZ Lysozyme MYO Myoglobin NaCl Sodium chloride NaClO4 Sodium perchlorate NaOH Sodium hydroxide i NC Nitrocellulose PANT Pantothenic acid PBS Phosphate buffer saline PCD Particle charge detector PDADMAC Poly(diallyldimethylammonium chloride) PDL Poly(D-lysine) PDI Polydispersity index PEC Polyelectrolyte complex nanoparticle PEC-0.66 Positively charged polyelectrolyte complex nanoparticle (n-/n+ = 0.66) PEC-1.50 Negatively charged polyelectrolyte complex nanoparticle (n-/n+ = -

ADVISORY NOTE Strasbourg, France, October 8Th 2009
ADVISORY NOTE Strasbourg, France, October 8th 2009 Details on SHIP-In R&D project revealed, a world-class project for French Research supported by the Alsace BioValley Cluster The SHIP-In research-and-development project has a single goal: developing new and innovative cellular systems for the biopharmaceutical industry. It received the label of the Alsace BioValley cluster in Alsace, France, in November 2008 and is co-sponsored by France’s Cancer Bio Santé and Medicen research poles. The pharmaceutical industry’s development of new therapeutic molecules requires the development and utilization of experimental cellular models that are both efficient and reliable. For a long time, the only possibility was to use stem cells extracted from human embryos. Apart from the difficulty of ensuring their availability, the use of such cells is also circumscribed by stringent ethical and regulatory constraints, which considerably hamper and slow the discovery of new medicines. Today, a new scientific approach offers an alternative to the use of embryonic stem cells, which is to start with adult human cells and reprogram them as multi-purpose cells. The SHIP-In project consists in optimizing this new technology to cater for the demands of the biopharmaceutical industry. The aim is to provide pharmaceutical and cosmetics companies with an unlimited supply of human cells which meet quality criteria that are not available today. Eventually, SHIP-In should facilitate the discovery of new molecules and generally speed up the therapeutic innovation process. “SHIP-In presents a unique opportunity for participating in the development of scientific advances that have been unanimously hailed at a world level,” declared Nicolas Carboni, the Director of the Alsace BioValley cluster, a partner in the project. -

Polyelectrolyte Complex: a Pharmaceutical Review
Review Article Polyelectrolyte Complex: A Pharmaceutical Review Dakhara SL, Anajwala CC Department of Pharmaceutics, Bhagwan Mahavir College of Pharmacy, Surat - 395 017, Gujarat, India ar T ic L E I NF O A bs T rac T Article history: This review work gives a lot of information on polyelectrolyte complexes (PECs). The complex Received 21 April 2010 formed is generally applied in different dosage forms for the formulation of stable aggregated Accepted 2 May 2010 macromolecules. Many properties like diffusion coefficient, chain conformation, viscosity, Available online 07 January 2011 polarizability, miscibility, etc., are drastically changed due to the introduction of a polyelectrolyte. Keywords: The formation of PECs is influenced not only by chemical properties like stereochemical fitting, Beads their molecular weight, charge densities, etc. but also by secondary experimental conditions In vitro release like concentration of polyelectrolytes prior to mixing, their mixing ratio, ionic strength of the Polyelectrolyte complex solution, mixing order, etc. The formation of PECs is described in this article and it is divided into Swelling three main classes, i.e., primary complex formation, formation process within intracomplexes and intercomplex aggregation process. There are different types of PECs obtained according to binding agents such as polymers, proteins, surfactants, drugs, etc. Other factors which affect the formation of PECs are also discussed. There are a number of pharmaceutical applications of polyelectrolytes, such as in controlled -

Stability of ,Aqueous a =Al203 Suspensions with Poly(Methacry1ic
J. Am. Cerum. SOC., 71 14) 250-55 (1988) Stability of ,Aqueous a =Al203Suspensions with Poly(methacry1ic acid) Polyelectrolyte JOSEPH CESARANO III* and ILHAN A. AKSAY* Department of Materials Science and Engiineering, College of Engineering, University of Washington, Seattle, Washington 98 195 ALAN BLEIER* Metals and Ceramics Division, Oak Ridge National Laboratory,* Oak Ridge, Tennessee 3783 1 Stability of aqueous a-A1,O3 suspensions with Na+ salt of have a substantial surface charge of the same sign so that irre- poly(methacry1ic acid) (PMAA-Na) polyelectrolyte was studied versible agglomeration is prevented.' In general, ceramic sus- as a function of pH. At a given pH, the transition from the pensions can be stabilized electrostatically, but improvement of the flocculated to the dispersed state corresponded to the ad- suspensions to better meet the requirements necessary for ceramic sorption saturation limit of the powders by the PMAA. As the processing is possible by incorporating polymeric additives. pH was decreased, the adsorption saturation limit increased Industrial experience shows, for instance, that in highly concen- until insolubility and charge neutralizatioin of the PMAA was trated oxide suspensions, problems related to high viscosity, aging, approached. The critical amount of PMAA required to achieve and processing of multiphase systems can be drastically reduced by stability is outlined in a stability map. using polyelectrolytes as dispersants or deflocc~lants.~.~However, in spite of these advantages in using polyelectrolytes to stabilize suspensions, a great deal of misunderstanding exists in the general ceramic community as to the fundamental roles of these polymeric I. Introduction additives. Thus, this investigation was designed to elucidate the OR many applications in ceramic processing it is desirable to mechanisms of polyelectrolyte stabilization and to relate them to F sinter at relatively low temperatures and to obtain fully dense the chemistry of the powder surface and the polymer additive. -

UNIVERSITY of CALIFORNIA Los Angeles Phase Behavior of Particle
UNIVERSITY OF CALIFORNIA Los Angeles Phase Behavior of Particle-Polyelectrolyte Complexes A thesis submitted in partial satisfaction of the requirements for the degree Master of Science in Chemical Engineering by John E. Neilsen 2019 ABSTRACT OF THE THESIS Phase Behavior of Particle-Polyelectrolyte Complexes by John Neilsen Master of Science in Chemical Engineering University of California, Los Angeles, 2019 Professor Samanvaya Srivastava, Chair The phase behavior of particle-polyelectrolyte complexes was systematically studied using a model system comprising oppositely charged silica nanoparticles and poly(allylamine) hydrochloride (PAH) polycations. Phase behaviors of aqueous mixtures of silica nanoparticles and PAH were elucidated over a wide parameter space of particle and polyelectrolyte concentrations as well as solution pH. Trends in phase behaviors were analyzed to create a fundamental understanding of the fundamental properties that govern the complexation of these oppositely charged species. ii The thesis of John Neilsen is approved. Vasilios Manousiouthakis Junyoung O. Park Samanvaya Srivastava, Committee Chair University of California, Los Angeles 2019 iii Contents 1. Introduction……………………………………………………..………………….…….…..….…..…1 1.1 Aqueous Particle-Polyelectrolyte Self-Assemblies…………..…….....………….....….….1 1.2 Biological Significance …………..……………………..…...….…...…......…….…………..2 1.3 Technological Applications…………..………………….……......……………...………....2 2. Background……………………………………………...………………….……………………..……5 2.1 The Voorn-Overbeek Theory……….………………….…….……………….……….……6 -

A Year at the Cnrs
2017 A YEAR AT THE CNRS in Alsace 2017 A YEAR AT THE CNRS IN ALSACE is a regional addition to the activity report sommaire 6 > 10 11 12 > 13 2017, a year at the CNRS THE LIVING WORLD SOCIÉTÉSSOCIETIES MATIÈREMATTER CNRS délégation Alsace 23 rue du Lœss - BP 20 67037 Strasbourg cedex 03 88 10 63 01 2 > 3 2017 in figures www.alsace.cnrs.fr 3 Editorial Patrice Soullie, Publisher Antoine Petit Regional Delegate Editorial director Patrice Soullie Chief editor Céline Delalex-Bindner Scientific committee Dominique Badariotti Frédéric Leroux 4 > 5 Rémi Barillon Frédéric Masson Highlights Christine Brunel Sylviane Muller Pierre-Alain Duc Jean-Serge Rémy Vincente Fortier Vincent Roucoules 18 > 19 Jean-Luc Galzi Bertrand Séraphin Science without borders Christian Gauthier Coordination, writing and iconography William Rowe-Pirra Layout and realisation Olivier Fély 20 Graphic design Céline Hein List of laboratories > Acknowledgements : , 14 15 Anne Bresson Pascaline Toutois 1614 17 Photo de couverture : Cover page: An artistic interpretation of the SAGA complex emerging from the nucleoplasmic sea while 21 grabbing a chromatin thread. Glossary ENGINEERING AND EARTHTERRE AND ET © Jonathon Broughton THE UNIVERSE Table of contents pictures : © CNRS Photothèque DIGITAL SCIENCES ENVIRONNEMENTENVIRONMENT Download this document online at : www.alsace.cnrs.fr | French version available Legal deposit : september 2018 - ISSN : 2270-4876 ÉCO VALO This pictogram indicates that the CNRS is economically involved. A number of facts published in this report, results of scientific equipment*, came to be thanks to the support of the European Union, the Région Grand Est, the Conseils departmentaux du Bas-Rhin et du Haut-Rhin, the Eurométropole de Strasbourg and the Mulhouse Alsace Agglomeration, Somes words are defined as well as numerous nonprofit partners.