Short Communication Comparison of Efficacy of Two
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparison of the Costs for Treating Central Precocious Puberty During the First Year with Monthly Leuprolide Acetate Injectable and Histrelin Acetate Implant
A Comparison Of The Costs For Treating Central Precocious Puberty During The First Year With Monthly Leuprolide Acetate Injectable And Histrelin Acetate Implant B F Banahan III1, Mayo K2, Summers KH2 1 Center for Pharmaceutical Marketing and Management and Department of Pharmacy Administration, University of Mississippi, University, MS 2 Health Outcomes & PharmacoEconomics, Endo Pharmaceuticals, Inc., Chadds Ford, PA Estimated Annual Cost of Treatment if all PTs Treated With . BACKGROUND METHODS MEDICAID MODEL Lupron SUPPRELIN LA Product costs $ 1,770,201 $ 1,515,188 Two retrospective cohort studies were conducted using datasets derived from the Office visit costs $ 79,896 $ 14,000 Puberty results when secretion of gonadotropin releasing hormone (GnRH) is initiated 100% compliance with Lupron treatment 1 Implant procedures $ 91,400 and the hypothalamic-pituitary-gonadal axis is activated. During puberty, the brain Thomas Reuter’s MarketScan© Multi-State Medicaid Database (2003-2007) and the (All patients receive 13+ treatments in year) MarketScan© Commercial Database (2005-2009). A probabilistic patient flow model Lab/xray for monitoring $ 53,900 $ 37,500 produces GnRH through a complex process. GnRH causes increases in other TOTAL COST TO PAYER $ 1,903,997 $ 1,658,088 hormones like luteinizing hormone (LH) and follicle stimulating hormone (FSH). It is was developed using estimates for treatment patterns and costs for products, office Normal Compliance with Lupron: Lupron SUPPRELIN LA these hormones that cause the ovaries to produce estrogen and the testicles to visits, and monitoring therapy. Patients with < 13 treatments per year Product costs $ 1,572,921 $ 1,515,188 Quality of Care ofCare Quality % of TXd PTs: 53% Aver. -

Hormones and Breeding
IN-DEPTH: REPRODUCTIVE ENDOCRINOLOGY Hormones and Breeding Carlos R.F. Pinto, MedVet, PhD, Diplomate ACT Author’s address: Theriogenology and Reproductive Medicine, Department of Veterinary Clinical Sciences, College of Veterinary Medicine, The Ohio State University, Columbus, OH 43210; e-mail: [email protected]. © 2013 AAEP. 1. Introduction affected by PGF treatment to induce estrus. In The administration of hormones to mares during other words, once luteolysis takes place, whether breeding management is an essential tool for equine induced by PGF treatment or occurring naturally, practitioners. Proper and timely administration of the events that follow (estrus behavior, ovulation specific hormones to broodmares may be targeted to and fertility) are essentially similar or minimally prevent reproductive disorders, to serve as an aid to affected (eg, decreased signs of behavioral estrus). treating reproductive disorders or hormonal imbal- Duration of diestrus and interovulatory intervals ances, and to optimize reproductive efficiency, for are shortened after PGF administration.1 The example, through induction of estrus or ovulation. equine corpus luteum (CL) is responsive to PGF These hormones, when administered exogenously, luteolytic effects any day after ovulation; however, act to control the duration and onset of the different only CL Ͼ5 days are responsive to one bolus injec- stages of the estrous cycle, specifically by affecting tion of PGF.2,3 Luteolysis or antiluteogenesis can duration of luteal function, hastening ovulation es- be reliably achieved in CL Ͻ5 days only if multiple pecially for timed artificial insemination and stimu- PGF treatments are administered. For that rea- lating myometrial activity in mares susceptible to or son, it became a widespread practice to administer showing delayed uterine clearance. -

Download Article As
FOCUS > EQUINE FOCUS < OVINE FOCUS > EQUINE Inducing timed ovulation in the mare Susan Salter BSc Hons BVM&S MRCVS and Jonathon Pycock BVetMed PhD DESM MRCVS compare and contrast various ovulating agents used to induce ovulation in mares at breeding, highlighting the advantages and disadvantages, effi cacy and welfare implications associated with each Weatherbys documented 14,747 active thoroughbred hCG was used on subsequent cycles. They also showed broodmares in Ireland in 2019, almost twice as many as that younger mares were more likely to ovulate within 48 the UK which recorded 8,571. Ireland is the third biggest hours than older mares when given hCG. Repeated use of producer of thoroughbreds in the world after Australia and hCG is, therefore, associated with decreased reliability in the USA.1 inducing timed ovulation and e icacy declines significantly In addition, it is estimated that there are around 15,000 active with increased mare age making it unreliable for use in breeders in the sport horse sector. 2 In order to maximise the older mares.3 In Ireland, hCG is still used since the deslorelin e iciency of breeding, it is essential that timing of ovulation implant has labour, cost, welfare and safety implications. can be manipulated e ectively. It is also imperative that attempts to manipulate timing of ovulation are not associated DESLORELIN ACETATE – THE IMPLANT AND THE with subsequent delays in return to oestrus. The current INJECTABLE Covid-19 crisis presents additional challenges and pressures Deslorelin is a gonadotrophin releasing hormone (GnRH) of balancing the economic imperative to continue equine receptor agonist. -

(CERCA), Ecole Nationale Vétérinaire D'alfort
Rev. Bras. Reprod. Anim., Belo Horizonte, v.35, n.2, p.210-216, abr./jun. 2011. Disponível em ww.cbra.org.br. The use of GnRH agonists implants in bitches and queens Utilização de implantes de agonistas do GnRH em cadelas e gatas A. Fontbonne1, E. Fontaine Centre d’Etude en Reproduction des Carnivores (CERCA), Alfort Veterinary College, Paris, France. 1Corresponding author: [email protected] Abstract GnRH (gonadotrophin releasing hormone) is a key hormone of reproductive function in mammals; agonist forms have been largely developed, and data concerning their use in small animal reproduction are now abundant. GnRH agonists act by a two-step mechanism. First, their agonist properties on the pituitary will cause marked LH (luteinizing hormone) and FSH (follicle-stimulating hormone) secretion into the bloodstream, accompanied by an increase in the concentrations of sex steroid hormones. Then, in case of constant administration, GnRH agonists will lead to pituitary desensitization, and FSH and LH levels will collapse. These two effects have been widely documented, and these compounds have many potential benefits in a clinical context, capitalizing both on their stimulating and sterilizing effects. Keywords: bitch, deslorelin, GnRH agonists, queen. Resumo O hormônio liberador de gonadotrofinas (GnRH) é um hormônio chave na função reprodutiva dos mamíferos. Formas agonistas têm sido amplamente desenvolvidas e atualmente existem muitas informações sobre sua utilização na reprodução de pequenos animais. Os agonistas do GnRH atuam por meio de um mecanismo que envolve duas etapas. Inicialmente, suas propriedades agonistas irão causar secreção marcante de hormônio luteinizante (LH) e folículo-estimulante (FSH) pela hipófise, acompanhado pelo aumento das concentrações dos hormônios esteróides sexuais. -

Use of a Gonadotropin Releasing Hormone Agonist Implant
Use of a Gonadotropin Releasing Hormone Agonist Implant Containing 4.7 mg Deslorelin for Medical Castration in Male Ferrets (Mustela putorius furo) Bulliot Christophe, DVM1 Mentré Véronique, DVM2 Berthelet Adeline, DVM3 Navarro Christelle, DVM4 Bidaud Alice, DVM4 1 Corresponding author: Clinique Vétérinaire Exotic Clinic, 38 rue Robert Cousin, 77176 Nandy, France. E-mail: [email protected] 2 Clinique Vétérinaire de la Patte d’Oie, 155 Bd Victor Bordier, 95370 Montigny les Cormeilles, France. E-mail: [email protected] 3 Clinique Vétérinaire Exotic Clinic, 38 rue Robert Cousin, 77176 Nandy, France. E-mail: [email protected] 4 Virbac, 13ème rue - LID, 06511 Carros, France. Conflict of interest: the study was funded by Virbac. KEY WORDS: Ferret, Deslorelin, tent, for medical castration. This study is the Gonadotropin, GnRH, medical castration first evaluation of a GnRH-agonist implant containing 4.7 mg deslorelin for medical ABSTRACT castration in male ferrets with an assessment Sterilization of ferrets (Mustela putorius of the duration of infertility over a 3-year furo) is a common practice. Male ferrets, follow-up. Twenty-nine intact male ferrets unlike females, don’t need castration for in rut were implanted and were used for medical reasons, but are frequently neutered tolerance evaluation. Infertility was assessed to prevent reproduction and reduce their in 27 ferrets by evaluating their testosterone musky odor and aggressive territorial behav- concentrations, testis size and musky odor. ior. The search for an alternative to surgical Our results indicated that infertility was in- castration is an important goal and challenge duced within 6 weeks post-implantation and in this species. -

DESCRIPTION Vantas™ (Histrelin Implant) Is a Sterile Non-Biodegradable, Diffusion-Controlled Reservoir Drug Delivery System De
Vantas™ (histrelin implant) PACKAGE INSERT DESCRIPTION Vantas™ (histrelin implant) is a sterile non-biodegradable, diffusion-controlled reservoir drug delivery system designed to deliver histrelin continuously for 12 months upon subcutaneous implantation. The Vantas implant contains 50 mg of histrelin acetate. Histrelin acetate is a synthetic nonapeptide analogue of the naturally occurring gonadotropin releasing hormone (GnRH) or luteinizing hormone releasing hormone (LH-RH). The sterile Vantas implantation device (provided with the implant) is used to insert the implant subcutaneously in the inner aspect of the upper arm. After 12 months, the implant must be removed. At the time the implant is removed, another implant may be inserted to continue therapy. The sterile Vantas ™ implant consists of a 50-mg histrelin acetate drug core inside a non- biodegradable, 3 cm by 3.5 mm cylindrically shaped hydrogel reservoir (Figure A). The drug core also contains the inactive ingredient stearic acid NF. The hydrogel reservoir is a hydrophilic polymer cartridge composed of 2-hydroxyethyl methacrylate, 2-hydroxypropyl methacrylate, trimethylolpropane trimethacrylate, benzoin methyl ether, Perkadox-16, and Triton X-100. The hydrated implant is packaged in a glass vial containing 2.0 mL of 1.8% NaCl solution. The implant is primed for release of the drug upon insertion. Figure A. Vantas Histrelin Implant diagram (not to scale) Hydrogel Reservoir Drug Formulation Histrelin acetate is chemically described as 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L- tyrosyl-Nt-benzyl-D-histidyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt) [C66H86N18O12. (1.7-2.8 moles) CH3COOH, (0.6-7.0 moles) H2O], with the molecular weight of 1443.70 (or 1323.50 as histrelin base. -

2018 Summary of Major Modifications and Explanatory Notes
SUMMARY OF MAJOR MODIFICATIONS AND EXPLANATORY NOTES 2018 PROHIBITED LIST Substances and methods prohibited at all times (In- and Out-of-Competition) Prohibited Substances S1 ANABOLIC AGENTS S3 BETA-2-AGONISTS • Dihydrotestosterone was renamed to its International • Dosing parameters of salbutamol were revised to make Non-proprietary Name (INN) (androstanolone). it clear that divided doses of salbutamol may not exceed 1-androsterone (3α-hydroxy-5α-androst-1-ene-17-one) 800 micrograms over any 12 hours (see figure). was added in S1.a as an example of exogenous anabolic steroid. Inhaled salbutamol – max. 1600 mcg over 24 hours But not to exceed 800 mcg over any 12 hours • LGD-4033 and RAD140 were added as further examples 0 12 24 of SARMs. Any 12 hour period: max. 800 mcg PEPTIDE HORMONES, GROWTH S2 FACTORS, RELATED SUBSTANCES 200 mcg 200 mcg 200 mcg 200 mcg 200 mcg 200 mcg 200 mcg 200 mcg AND MIMETICS • For clarity and accuracy Section S2 was reorganized. • Tulobuterol was added as an example. • ARA290 was removed as an example in this section • The statement on the urinary thresholds was improved. because current literature suggests it does not meet inclusion criteria. • Deslorelin, goserelin, nafarelin and triptorelin were HORMONE AND METABOLIC added as examples of 2.1. S4 MODULATORS • Growth Hormone fragments were included in 2.3 with • Clomifene is now stated by its INN. AOD-9604 and hGH 176-191 added as examples; CJC-1293 was added as example of GHRH and • In the absence of an INN, the IUPAC name of GW1516, tabimorelin as a further example of GH secretagogue. -

PE2572 Puberty Blockers
Puberty Blockers What are puberty Puberty blockers are medicines that block puberty-related hormones that blockers? make your body go through puberty. Starting puberty blockers is a decision that is different for everyone. To make the most informed decision, this handout is meant to help you understand: • What is puberty? • What do puberty blockers do? • What are the changes that will happen to my body? • What are the benefits, risks and costs involved? We will work with you to support the decision that is best for you. You can view a video about puberty blockers at seattlechildrens.org/gender. How does puberty Puberty is the process the body goes through to become capable of begin? making a baby (reproduction), as well as reach adult size and brain development. Puberty starts when your brain tells your pituitary gland to start releasing puberty-related hormones. This happens at different ages for different people. During this time, your body starts to increase the amount of certain puberty-related hormones (Luteinizing Hormone (LH) and Follicle- Stimulating Hormone (FSH). This causes your testicles to start producing testosterone or your ovaries start producing estrogen. These hormones do not cause acne, pubic or armpit hair – those are caused by other hormones. Body changes in • Testicle growth (this improves the body’s ability to make testosterone) people with testicles • Penis growth (without puberty • Pubic hair blockers) • Increased acne, increased armpit and facial hair • Rapid growth (growth spurt) • Voice changes (deepens) Body changes in • Breast changes people with ovaries • Changes in body shape, including fuller hips (without puberty • Menstrual periods start (usually more than 2 years after breast changes blockers) begin) 1 of 6 To Learn More Free Interpreter Services • Adolescent Medicine • In the hospital, ask your nurse. -
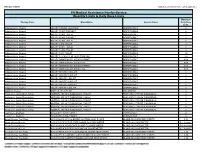
Quantity Limits/Daily Dose Limits
Effective 07/20/21 Alphabetical by Brand Name (when applicable) PA Medical Assistance Fee-for-Service Quantity Limits & Daily Dose Limits Maximum Therapy Class Brand Name Generic Name Daily Dose Limit Antipsychotics, Atypical ABILIFY 1 MG/ML SOLUTION ARIPIPRAZOLE 25 Antipsychotics, Atypical ABILIFY 10 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 10 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 15 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 15 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 2 MG TABLET ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 20 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 30 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 5 MG TABLET ARIPIPRAZOLE 1.5 Antipsychotics, Atypical ABILIFY 9.75 MG/1.3 ML INJECTION VIAL ARIPIPRAZOLE 3.9 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MYCITE 10 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 15 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 2 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 20 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 30 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 5 MG KIT ARIPIPRAZOLE 1 Antivirals, Herpes ABREVA 10% -
![(12) United States Patent (10) Patent N0.: US 7,309,689 B2 Trigg Et A]](https://docslib.b-cdn.net/cover/7219/12-united-states-patent-10-patent-n0-us-7-309-689-b2-trigg-et-a-1507219.webp)
(12) United States Patent (10) Patent N0.: US 7,309,689 B2 Trigg Et A]
US007309689B2 (12) United States Patent (10) Patent N0.: US 7,309,689 B2 Trigg et a]. (45) Date of Patent: *Dec. 18, 2007 (54) SUSTAINED PEPTIDE-RELEASE 5,256,649 A 10/1993 Le Fur et al. FORMULATION 5,340,585 A 8/1994 Pike 5,372,996 A 12/1994 Labrie ....................... .. 514/15 (75) Inventors: Timothy Elliot Trigg, WarraWee (AU); 5,573,781 A 11/1996 Brown et a1. ............. .. 424/484 John Desmond Walsh, Curl Curl (AU); 5,925,619 A 7/1999 Walsh Paul Adam Schober, Beacon Hill (AU) 6,337,318 B1 1/2002 Trigg et al. ................. .. 514/15 FOREIGN PATENT DOCUMENTS (73) Assignee: Peptech Limited, North Ryde (AU) AU A-41059/85 10/1985 ( * ) Notice: Subject to any disclaimer, the term of this EP 0 158 277 10/1985 patent is extended or adjusted under 35 EP 0 254 693 1/1988 U.S.C. 154(b) by 268 days. EP 0 645 136 A2 3/1995 GB 2 052 258 1/1981 JP 62-192327 8/1987 This patent is subject to a terminal dis WO 86/04503 8/1986 claimer. WO 92/18107 10/1992 W0 WO 93/07833 4/1993 WO 92/15722 8/1993 (21) Appl. No.: 10/808,504 WO 96/34012 10/1996 (22) Filed: Mar. 25, 2004 W0 WO 97/00693 1/1997 (65) Prior Publication Data OTHER PUBLICATIONS US 2004/0180832 A1 Sep. 16, 2004 Liu et a1; “E?cects of PituitaryiTesticular Axis Suppression in Utero and during . ”, Journal ofEndocrinology and Related US. Application Data Metabolism, vol. 73, No. -

DEAR PHYSICIAN: This Letter Is Being Provided As a Sample to Help You with Your Payor Interactions Concerning Reimbursement
DEAR PHYSICIAN: This letter is being provided as a sample to help you with your payor interactions concerning reimbursement for the administration of SUPPRELIN® LA (histrelin acetate) subcutaneous implant. Use of this document does not guarantee coverage or reimbursement. As a healthcare professional, you are solely responsible for providing accurate information to third-party payors. If there is any information in this document that does not accurately reflect your practices, it should be modified to appropriately represent your particular circumstances. INDICATION • SUPPRELIN® LA (histrelin acetate) subcutaneous implant is indicated for the treatment of children with central precocious puberty (CPP). • Children with CPP (neurogenic or idiopathic) have an early onset of secondary sexual characteristics (earlier than 8 years of age in females and 9 years of age in males). They also show a significantly advanced bone age that can result in diminished adult height attainment. • Prior to initiation of treatment, a clinical diagnosis of CPP should be confirmed by measurement of blood concentrations of total sex steroids, luteinizing hormone (LH) and follicle stimulating hormone (FSH) following stimulation with a GnRH analog, and assessment of bone age versus chronological age. Baseline evaluations should include height and weight measurements, diagnostic imaging of the brain (to rule out intracranial tumor), pelvic/testicular/adrenal ultrasound (to rule out steroid secreting tumors), human chorionic gonadotropin levels (to rule out a chorionic gonadotropin secreting tumor), and adrenal steroids to exclude congenital adrenal hyperplasia. IMPORTANT SAFETY INFORMATION ABOUT SUPPRELIN® LA • SUPPRELIN® LA is contraindicated in patients who are hypersensitive to gonadotropin releasing hormone (GnRH) or GnRH agonist analogs and in females who are or may become pregnant while receiving the drug. -

Erleada (Apalutamide)
Market Applicability Market GA KY MD NJ NY WA Applicable X X X X X X Erleada (apalutamide) Override(s) Approval Duration Prior Authorization 1 year Quantity Limit Medications Quantity Limit Erleada (apalutamide) May be subject to quantity limit APPROVAL CRITERIA Requests for Erleada (apalutamide) may be approved if the following criteria are met: I. Individual is diagnosed with one of the following: A. Individual has a diagnosis of non-metastatic castration-resistant* prostate cancer (nmCRPC); OR B. Individual has a diagnosis of metastatic castration-sensitive prostate cancer (mCSPC) AND II. One of the following: A. Individual is concomitantly receiving a gonadotropin-releasing hormone (GnRH) analog (e.g. Lupron (leuprolide, Zoladex (goserelin), Trelstar (triptorelin), Vantas (histrelin), Firmagon (degarelix); OR B. Individual has had a bilateral orchiectomy. *Castration-resistant refers to either surgical or medically induced methods. Medically induced methods include luteinizing hormone-releasing hormone (LHRH) agonists (such as leuprolide, goserelin) or LHRH antagonists (such as degarelix). Key References: 1. Clinical Pharmacology [database online]. Tampa, FL: Gold Standard, Inc.: 2020. URL: http://www.clinicalpharmacology.com. Updated periodically. 2. DailyMed. Package inserts. U.S. National Library of Medicine, National Institutes of Health website. http://dailymed.nlm.nih.gov/dailymed/about.cfm. Accessed: April 19, 2020. PAGE 1 of 2 09/11/2020 CRX-ALL-0595-20 New Program Date 04/09/2018 This policy does not apply to health plans or member categories that do not have pharmacy benefits, nor does it apply to Medicare. Note that market specific restrictions or transition-of-care benefit limitations may apply. Market Applicability Market GA KY MD NJ NY WA Applicable X X X X X X 3.