Amount of Substance and the Mole
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

About SI Units
Units SI units: International system of units (French: “SI” means “Système Internationale”) The seven SI base units are: Mass: kg Defined by the prototype “standard kilogram” located in Paris. The standard kilogram is made of Pt metal. Length: m Originally defined by the prototype “standard meter” located in Paris. Then defined as 1,650,763.73 wavelengths of the orange-red radiation of Krypton86 under certain specified conditions. (Official definition: The distance traveled by light in vacuum during a time interval of 1 / 299 792 458 of a second) Time: s The second is defined as the duration of a certain number of oscillations of radiation coming from Cs atoms. (Official definition: The second is the duration of 9,192,631,770 periods of the radiation of the hyperfine- level transition of the ground state of the Cs133 atom) Current: A Defined as the current that causes a certain force between two parallel wires. (Official definition: The ampere is that constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section, and placed 1 meter apart in vacuum, would produce between these conductors a force equal to 2 × 10-7 Newton per meter of length. Temperature: K One percent of the temperature difference between boiling point and freezing point of water. (Official definition: The Kelvin, unit of thermodynamic temperature, is the fraction 1 / 273.16 of the thermodynamic temperature of the triple point of water. Amount of substance: mol The amount of a substance that contains Avogadro’s number NAvo = 6.0221 × 1023 of atoms or molecules. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

2.1.3 Amount of Substance the Mole Is the Key Concept for Chemical Calculations
2.1.3 Amount of substance The mole is the key concept for chemical calculations DEFINITION: The mole is the amount of substance in grams that has the same number of particles as there are atoms in 12 grams of carbon-12. DEFINITION: Relative atomic mass is the average mass of one atom compared to one twelfth of the mass of one atom of carbon-12 DEFINITION: Molar Mass is the mass in grams of 1 mole of a substance and is given the unit of g mol-1 Molar Mass for a compound can be calculated by adding up the mass numbers(from the periodic table) of each element in the compound eg CaCO3 = 40.1 + 12.0 +16.0 x3 = 100.1 molar gas volume (gas volume per mole, units dm3 mol–1) . This is the volume of 1 mole of a gas at a given temperature and pressure. All gases have this same volume. At room pressure (1atm) and room temperature 25oC the molar gas volume is 24 dm3 mol–1 Avogadro's Constant There are 6.02 x 1023 atoms in 12 grams of carbon-12. Therefore explained in simpler terms 'One mole of any specified entity contains 6.02 x 1023 of that entity': 1 mole of copper atoms will contain 6.02 x 1023 atoms Avogadro's Constant can be 1 mole of carbon dioxide molecules will contain 6.02 x 1023 molecules used for atoms, molecules and 1 mole of sodium ions will contain 6.02 x 1023 ions ions For pure solids, liquids and gases Example 1: What is the amount, in mol, in 35.0g of CuSO4? amount = mass amount = mass/Mr Mr = 35/ (63.5 + 32 +16 x4) Unit of Mass: grams = 0.219 mol Unit of amount : mol Many questions will involve changes of units 1000 mg =1g 1000 g =1kg 1000kg = 1 tonne Significant Figures Give your answers to the same number of significant figures as the Example 2: What is the amount, in mol, in 75.0mg of number of significant figures for the CaSO4.2H2O? data you given in a question. -

2019 Redefinition of SI Base Units
2019 redefinition of SI base units A redefinition of SI base units is scheduled to come into force on 20 May 2019.[1][2] The kilogram, ampere, kelvin, and mole will then be defined by setting exact numerical values for the Planck constant (h), the elementary electric charge (e), the Boltzmann constant (k), and the Avogadro constant (NA), respectively. The metre and candela are already defined by physical constants, subject to correction to their present definitions. The new definitions aim to improve the SI without changing the size of any units, thus ensuring continuity with existing measurements.[3][4] In November 2018, the 26th General Conference on Weights and Measures (CGPM) unanimously approved these changes,[5][6] which the International Committee for Weights and Measures (CIPM) had proposed earlier that year.[7]:23 The previous major change of the metric system was in 1960 when the International System of Units (SI) was formally published. The SI is a coherent system structured around seven base units whose definitions are unconstrained by that of any other unit and another twenty-two named units derived from these base units. The metre was redefined in terms of the wavelength of a spectral line of a The SI system after the 2019 redefinition: krypton-86 radiation,[Note 1] making it derivable from universal natural Dependence of base unit definitions onphysical constants with fixed numerical values and on other phenomena, but the kilogram remained defined in terms of a physical prototype, base units. leaving it the only artefact upon which the SI unit definitions depend. The metric system was originally conceived as a system of measurement that was derivable from unchanging phenomena,[8] but practical limitations necessitated the use of artefacts (the prototype metre and prototype kilogram) when the metric system was first introduced in France in 1799. -
![Arxiv:0801.0028V1 [Physics.Atom-Ph] 29 Dec 2007 § ‡ † (People’S China Metrology, Of) of Lic Institute Canada National Council, Zhang, Research Z](https://docslib.b-cdn.net/cover/1910/arxiv-0801-0028v1-physics-atom-ph-29-dec-2007-%C2%A7-people-s-china-metrology-of-of-lic-institute-canada-national-council-zhang-research-z-651910.webp)
Arxiv:0801.0028V1 [Physics.Atom-Ph] 29 Dec 2007 § ‡ † (People’S China Metrology, Of) of Lic Institute Canada National Council, Zhang, Research Z
CODATA Recommended Values of the Fundamental Physical Constants: 2006∗ Peter J. Mohr†, Barry N. Taylor‡, and David B. Newell§, National Institute of Standards and Technology, Gaithersburg, Maryland 20899-8420, USA (Dated: March 29, 2012) This paper gives the 2006 self-consistent set of values of the basic constants and conversion factors of physics and chemistry recommended by the Committee on Data for Science and Technology (CODATA) for international use. Further, it describes in detail the adjustment of the values of the constants, including the selection of the final set of input data based on the results of least-squares analyses. The 2006 adjustment takes into account the data considered in the 2002 adjustment as well as the data that became available between 31 December 2002, the closing date of that adjustment, and 31 December 2006, the closing date of the new adjustment. The new data have led to a significant reduction in the uncertainties of many recommended values. The 2006 set replaces the previously recommended 2002 CODATA set and may also be found on the World Wide Web at physics.nist.gov/constants. Contents 3. Cyclotron resonance measurement of the electron relative atomic mass Ar(e) 8 Glossary 2 4. Atomic transition frequencies 8 1. Introduction 4 1. Hydrogen and deuterium transition frequencies, the 1. Background 4 Rydberg constant R∞, and the proton and deuteron charge radii R , R 8 2. Time variation of the constants 5 p d 1. Theory relevant to the Rydberg constant 9 3. Outline of paper 5 2. Experiments on hydrogen and deuterium 16 3. -

Properties of Matter
Properties of Matter Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution/Non- Commercial/Share Alike 3.0 Unported (CC BY-NC-SA) License (http://creativecommons.org/licenses/by-nc-sa/3.0/), as amended and updated by Creative Commons from time to time (the “CC License”), which is incorporated herein by this reference. -

PHYSICS of Materials (PHYS132)
PHYSICS of Materials (PHYS132) Dr Sergey Burdin (Oliver Lodge, Room 314, [email protected]) (based on earlier lectures by Prof. R. McGrath and Dr Joost Vossebeld) 7 topics (13 lectures) • Introduction: definitions, structure (2) • inter-atomic forces (2) • Thermal properties: States of matter, latent heat, thermal expansion (3) • Mechanical properties: elasticity (1) • Magnetic properties (2) • Electrical properties: band theory, semi-conductors (2) • Optical properties: colour (1) 3 question sheets: 10% of final mark for satisfactory submission. Fairly difficult but good practice for exam. 6 weeks × 3 hours – 1 = 17 hours (13 lectures + 3 problem sessions + 1) 1. Introduction to Materials > 1.1 Definitions 1 PHYSICS of Materials PHYS132 Books: (recommended, not required) Fundamentals of Physics (Haliday, Resnick, Walker): Electrical and magnetic properties of matter Properties of Matter (Flowers and Mendoza): Structure, interatomic potentials, thermal properties, mechanical properties Gases, Liquids and Solids (Tabor): Structure, interatomic potentials, thermal properties Properties of Materials (White): Optical properties 1. Introduction to Materials > 1.1 Definitions 2 PHYSICS of Materials PHYS132 General approach: Start with: MICROSCOPIC properties of matter: Structure of materials. Forces between atoms, molecules, ions. (Atomic Physics & Chemistry) To arrive at: MACROSCOPIC properties of matter: density, elasticity, latent heat, conductivity, etc. (Material science, Engineering, Applied physics) 1. Introduction to Materials > 1.1 Definitions 3 Section 1. Introduction to materials Topic 1.1 Definitions Atoms, Molecules, Ions. Mole, Molar Mass, Atomic mass Few example calculations §1. Introduction to Materials > 1.1 Definitions 4 Atoms, ions and molecules Macroscopic matter is made up of assemblies of atoms, ions and molecules He ATOMS n n The smallest particle of an ELEMENT consists of p p NUCLEUS (Z Protons + N Neutrons) surrounded by Z Electrons. -

The International System of Units (SI)
NAT'L INST. OF STAND & TECH NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 330 2001 Edition The International System of Units (SI) 4. Barry N. Taylor, Editor r A o o L57 330 2oOI rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -
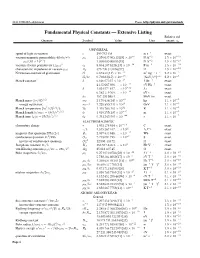
Fundamental Physical Constants — Extensive Listing Relative Std
2018 CODATA adjustment From: http://physics.nist.gov/constants Fundamental Physical Constants — Extensive Listing Relative std. Quantity Symbol Value Unit uncert. ur UNIVERSAL speed of light in vacuum c 299 792 458 m s−1 exact 2 −6 −2 −10 vacuum magnetic permeability 4pα¯h=e c µ0 1:256 637 062 12(19) × 10 NA 1:5 × 10 −7 −2 −10 µ0=(4p × 10 ) 1:000 000 000 55(15) NA 1:5 × 10 2 −12 −1 −10 vacuum electric permittivity 1/µ0c 0 8:854 187 8128(13) × 10 F m 1:5 × 10 −10 characteristic impedance of vacuum µ0c Z0 376:730 313 668(57) Ω 1:5 × 10 Newtonian constant of gravitation G 6:674 30(15) × 10−11 m3 kg−1 s−2 2:2 × 10−5 G=¯hc 6:708 83(15) × 10−39 (GeV=c2)−2 2:2 × 10−5 Planck constant∗ h 6:626 070 15 × 10−34 J Hz−1 exact 4:135 667 696 ::: × 10−15 eV Hz−1 exact ¯h 1:054 571 817 ::: × 10−34 J s exact 6:582 119 569 ::: × 10−16 eV s exact ¯hc 197:326 980 4 ::: MeV fm exact 1=2 −8 −5 Planck mass (¯hc=G) mP 2:176 434(24) × 10 kg 1:1 × 10 2 19 −5 energy equivalent mPc 1:220 890(14) × 10 GeV 1:1 × 10 5 1=2 32 −5 Planck temperature (¯hc =G) =k TP 1:416 784(16) × 10 K 1:1 × 10 3 1=2 −35 −5 Planck length ¯h=mPc = (¯hG=c ) lP 1:616 255(18) × 10 m 1:1 × 10 5 1=2 −44 −5 Planck time lP=c = (¯hG=c ) tP 5:391 247(60) × 10 s 1:1 × 10 ELECTROMAGNETIC elementary charge e 1:602 176 634 × 10−19 C exact e=¯h 1:519 267 447 ::: × 1015 AJ−1 exact −15 magnetic flux quantum 2p¯h=(2e) Φ0 2:067 833 848 ::: × 10 Wb exact 2 −5 conductance quantum 2e =2p¯h G0 7:748 091 729 ::: × 10 S exact −1 inverse of conductance quantum G0 12 906:403 72 ::: Ω exact 9 −1 Josephson constant 2e=h -

Molar Mass 1 Molar Mass
Molar mass 1 Molar mass In chemistry, the molar mass is a physical property. It is defined as the mass of a given substance (chemical element or chemical compound) divided by its amount of substance. The base SI unit for molar mass is kg/mol. However, for historical reasons, molar masses are almost always expressed in g/mol. As an example, the molar mass of water is approximately: M(H O) ≈ 18 g⋅mol−1 2 Molar masses of elements The molar mass of atoms of an element is given by the atomic mass of the element multiplied by the molar mass constant, M −3 u = 1×10 kg/mol = 1 g/mol: M(H) = 1.007 97(7) × 1 g/mol = 1.007 97(7) g/mol M(S) = 32.065(5) × 1 g/mol = 32.065(5) g/mol M(Cl) = 35.453(2) × 1 g/mol = 35.453(2) g/mol M(Fe) = 55.845(2) × 1 g/mol = 55.845(2) g/mol. Multiplying by the molar mass constant ensures that the calculation is dimensionally correct: atomic weights are dimensionless quantities (i.e., pure numbers) whereas molar masses have units (in this case, grams/mole). Some elements are usually encountered as molecules, e.g. hydrogen (H 2), sulfur (S 8), chlorine (Cl 2). The molar mass of molecules of these elements is the molar mass of the atoms multiplied by the number of atoms in each molecule: M(H 2) = 2 × 1.007 97(7) × 1 g/mol = 2.015 88(14) g/mol M(S 8) = 8 × 32.065(5) × 1 g/mol = 256.52(4) g/mol M(Cl 2) = 2 × 35.453(2) × 1 g/mol = 70.906(4) g/mol. -

Chapter 11: Gases
CHAPTER 11 GASES t’s Monday morning, and Lilia is walking out of the chemistry building, thinking 11.1 Gases and Their about the introductory lecture on gases that her instructor just presented. Dr. Properties Scanlon challenged the class to try to visualize gases in terms of the model she described, so Lilia looks at her hand and tries to picture the particles in the air 11.2 Ideal Gas Calculations bombarding her skin at a rate of 1023 collisions per second. Lilia has high hopes that a week of studying gases will provide her with answers to the questions her older brothers 11.3 Equation and sisters posed to her the night before at a family dinner. Stoichiometry and When Ted, who is a mechanic for a Formula One racing team, learned that Lilia Ideal Gases was going to be studying gases in her chemistry class, he asked her to find out how to 11.4 Dalton’s Law of calculate gas density. He knows that when the density of the air changes, he needs to Partial Pressures adjust the car’s brakes and other components to improve its safety and performance. John, who is an environmental scientist, wanted to be reminded why balloons that carry his scientific instruments into the upper atmosphere expand as they rise. Amelia is an artist who recently began to add neon lights to her work. After bending the tubes into the desired shape, she fills them with gas from a high pressure cylinder. She wanted to know how to determine the number of tubes she can fill with one cylinder. -

Chapter 16. a Macroscopic Description of Matter Chapter 16. A
10/12/10 Chapter 16. A Macroscopic Description of Chapter 16. A Macroscopic Description of Matter Matter Macroscopic systems are characterized as being either Topics: solid, liquid, or gas. These • Solids, Liquids, and Gases are called the phases of • Atoms and Moles matter, and in this chapter Temperature we’ll be interested in when • and how a system changes • Phase Changes from one phase to another. • Ideal Gases Chapter Goal: To learn the • Ideal-Gas Processes characteristics of macroscopic systems. Copyright © 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley. Copyright © 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley. State Variables Density The ratio of a system’s mass to its volume is called the • Parameters for macroscopic description of mass density, or sometimes simply “the density.” matter • Volume, pressure, mass, mass density, thermal energy, moles, number density, temperature The SI units of mass density are kg/m3. In this chapter we’ll use an uppercase M for the system mass and lowercase m for the mass of an atom. Copyright © 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley. Copyright © 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley. Atoms and Moles • The mass of an atom is determined primarily by its most massive constituents, the protons and neutrons in its nucleus. • The sum of the number of protons and neutrons is called the atomic mass number A. • The atomic mass scale is established by defining the mass of 12C to be exactly 12 u, where u is the symbol for the atomic mass unit. • The conversion factor between atomic mass units and kilograms is Copyright © 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley.