1 Lasers: Fundamentals, Types, and Operations Subhash Chandra Singh, Haibo Zeng, Chunlei Guo, and Weiping Cai
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Application of the Theory of Laser to Nitrogen Laser Pumped Dye Laser
SD9900039 AN APPLICATION OF THE THEORY OF LASER TO NITROGEN LASER PUMPED DYE LASER FATIMA AHMED OSMAN A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Physics. UNIVERSITY OF KHARTOUM FACULTY OF SCIENCE DEPARTMENT OF PHYSICS MARCH 1998 \ 3 0-44 In this thesis we gave a general discussion on lasers, reviewing some of are properties, types and applications. We also conducted an experiment where we obtained a dye laser pumped by nitrogen laser with a wave length of 337.1 nm and a power of 5 Mw. It was noticed that the produced radiation possesses ^ characteristic^ different from those of other types of laser. This' characteristics determine^ the tunability i.e. the possibility of choosing the appropriately required wave-length of radiation for various applications. DEDICATION TO MY BELOVED PARENTS AND MY SISTER NADI A ACKNOWLEDGEMENTS I would like to express my deep gratitude to my supervisor Dr. AH El Tahir Sharaf El-Din, for his continuous support and guidance. I am also grateful to Dr. Maui Hammed Shaded, for encouragement, and advice in using the computer. Thanks also go to Ustaz Akram Yousif Ibrahim for helping me while conducting the experimental part of the thesis, and to Ustaz Abaker Ali Abdalla, for advising me in several respects. I also thank my teachers in the Physics Department, of the Faculty of Science, University of Khartoum and my colleagues and co- workers at laser laboratory whose support and encouragement me created the right atmosphere of research for me. Finally I would like to thank my brother Salah Ahmed Osman, Mr. -

High-Power Solid-State Lasers from a Laser Glass Perspective
LLNL-JRNL-464385 High-Power Solid-State Lasers from a Laser Glass Perspective J. H. Campbell, J. S. Hayden, A. J. Marker December 22, 2010 Internationakl Journal of Applied Glass Science Disclaimer This document was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor Lawrence Livermore National Security, LLC, nor any of their employees makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or Lawrence Livermore National Security, LLC. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or Lawrence Livermore National Security, LLC, and shall not be used for advertising or product endorsement purposes. High-Power Solid-State Lasers from a Laser Glass Perspective John H. Campbell, Lawrence Livermore National Laboratory, Livermore, CA Joseph S. Hayden and Alex Marker, Schott North America, Inc., Duryea, PA Abstract Advances in laser glass compositions and manufacturing have enabled a new class of high-energy/high- power (HEHP), petawatt (PW) and high-average-power (HAP) laser systems that are being used for fusion energy ignition demonstration, fundamental physics research and materials processing, respectively. The requirements for these three laser systems are different necessitating different glasses or groups of glasses. -

HD DVD: Manufacturing Was Developed.This Recorder Is Equipped with a 257Nm Gas Laser (Frequency Doubled Ar+ Laser)
paper r& white d Six years ago, the LDM 3692 DUV recorder HD DVD: Manufacturing was developed.This recorder is equipped with a 257nm gas laser (frequency doubled Ar+ laser). All options with regards to future for- mats were still open at that time.The recorder features two recording spots, with a wobble The New Format option on both. This recorder is an adequate R&D tool to record HD DVD. BY DR. DICK VERHAART, from 740nm to 400nm. To read these smaller For HD DVD stamper manufacturing, a Singulus Mastering information structures, it is necessary to use recorder with a 266nm solid state laser was PETER KNIPS, blue diode lasers with a wavelength of 405nm developed. This system contains a stable and Singulus EMould instead of the 650nm red lasers used for CD easy to operate solid state laser, with a much DIETER WAGNER, and DVD. longer lifetime than the gas laser. As all pro- Singulus Technologies AG An advanced copy protection system will posed next-generation formats require only The third generation of optical disc formats is give better protection than what was avail- one spot, the system has a single recording set to arrive on the market by the end of this able for CD and DVD with mandatory serializ- spot. Spot deflection, required to create the year.As with Blu-ray Disc, the HD DVD format ing of each single HD DVD. The serialization groove wobble in the recordable and was developed to tremendously increase the will take place on the aluminum covered layer rewritable formats, is available as an option. -

Restricted Energy Transfer in Laser Desorption of High Molecular Weight Biomolecules
Scanning Microscopy Volume 5 Number 2 Article 3 4-20-1991 Restricted Energy Transfer in Laser Desorption of High Molecular Weight Biomolecules Akos Vertes University of Antwerp, Belgium, [email protected] Renaat Gijbels University of Antwerp, Belgium Follow this and additional works at: https://digitalcommons.usu.edu/microscopy Part of the Biology Commons Recommended Citation Vertes, Akos and Gijbels, Renaat (1991) "Restricted Energy Transfer in Laser Desorption of High Molecular Weight Biomolecules," Scanning Microscopy: Vol. 5 : No. 2 , Article 3. Available at: https://digitalcommons.usu.edu/microscopy/vol5/iss2/3 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Scanning Microscopy by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Scanning Microscopy, Vol. 5, No. 2, 1991 (Pages 317-328) 0891-7035/91$3.00+ .00 Scanning Microscopy International, Chicago (AMF O'Hare), IL 60666 USA RESTRICTED ENERGY TRANSFER IN LASER DESORPTION OF HIGH MOLECULAR WEIGHT BIOMOLECULES Akos Vertes* and Renaat Gijb els Departm ent of Chemistry, University of Antwerp (U.I.A.), Universiteitsplein 1, B-2610 Wilrijk (Belgium) (Received for publication November 15, 1990, and in revised form April 20, 1991) Abstract Introdu ction Producing ions from large molecules is of distin With the growing importan ce of biomedical investi guished importance in mass spectrometry. In our present gations in organic analysis the emphasis has been shift study we survey different laser desorption methods in ing to the detection and structure determination of ever view of their virtues and drawbacks in volatilization and larg er and more complex molecules. -

Ultrafast Fiber Lasers Enabled by Highly Nonlinear Pulse Evolutions
ULTRAFAST FIBER LASERS ENABLED BY HIGHLY NONLINEAR PULSE EVOLUTIONS A Dissertation Presented to the Faculty of the Graduate School of Cornell University in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy by Walter Pupin Fu August 2019 c 2019 Walter Pupin Fu ALL RIGHTS RESERVED ULTRAFAST FIBER LASERS ENABLED BY HIGHLY NONLINEAR PULSE EVOLUTIONS Walter Pupin Fu, Ph.D. Cornell University 2019 Ultrafast lasers have had tremendous impact on both science and applications, far beyond what their inventors could have imagined. Commercially-available solid-state lasers can readily generate coherent pulses lasting only a few tens of femtoseconds. The availability of such short pulses, and the huge peak in- tensities they enable, has allowed scientists and engineers to probe and manip- ulate materials to an unprecedented degree. Nevertheless, the scope of these advances has been curtailed by the complexity, size, and unreliability of such devices. For all the progress that laser science has made, most ultrafast lasers remain bulky, solid-state systems prone to misalignments during heavy use. The advent of fiber lasers with capabilities approaching that of traditional, solid-state lasers offers one means of solving these problems. Fiber systems can be fully integrated to be alignment-free, while their waveguide structure en- sures nearly perfect beam quality. However, these advantages come at a cost: the tight confinement and long interaction lengths make both linear and non- linear effects significant in shaping pulses. Much research over the past few decades has been devoted to harnessing and managing these effects in the pur- suit of fiber lasers with higher powers, stronger intensities, and shorter pulse durations. -

Wavelength-Tunable Ion Laser Systems
38Ch_AirCooledIonLsrs_f_v3.qxd 6/8/2005 11:19 AM Page 38.4 Diode-Pumped Solid-State Lasers Wavelength-Tunable Ion Laser Systems $ Selectable wavelengths ranging from violet to red SPECIFICATIONS: Air-Cooled Ion Lasers Air-Cooled $ Output power to 195 mW WAVELENGTH-TUNABLE ION LASER SYSTEMS $ Highest output power for its size Transverse Mode : TEM00 (except as noted) Longitudinal Mode Spacing : $ Switching power supplies with power-factor correction 35 (X)AP 321: 469 MHz $ CE, IEC, and CDRH compliant 35 (X)AP 431: 349 MHz These air-cooled ion laser systems are compact devices that pro- Coherence Length : ~10 cm duce high-quality, linearly polarized output that can be tuned over Polarization : Linear (vertical85°) with >250:1 extinction ratio a wide variety of wavelengths. The 35 LAP 321 and 35 MAP 321 are Pointing Stability : <30 mrad°C extremely compact argon-ion lasers, less than 15 inches in length Power Stability : 80.5% over a 2-hour period (excluding cables). The somewhat longer 35 LAP 431 and Optical Noise (%p-p @ <100 kHz / <1 MHz) : 35 MAP 431 are argon-ion lasers that produce as much as 195 mW Argon: 488 nm, 4 / 6; 514 nm, 4 / 6 Helium Cadmium Lasers multimode and up to 130 mW with a clean Gaussian TEM00 mode. Argon/Krypton: 488 nm, 5 / 7; 514 nm, 6 / 10; The model 35 KAP 431 is a mixed-gas (argon/krypton) laser with 568 nm, 3 / 6; 647 nm, 5 / 8 nine selectable wavelengths ranging from 476 nm (violet) to 676 nm Warm-up Time : <15 minutes from cold start (red). -

Book of Abstracts
Russian Academy of Sciences Institute of Problems of Chemical Physics RAS Joint Institute for High Temperatures RAS XIII International Conference on Physics of Non-Ideal Plasmas September 13 | 18, 2009, Chernogolovka, Russia Book of Abstracts Chernogolovka 2009 The book consists of the abstracts of oral and poster contributions to the XIII International Conference on Physics of Non-Ideal Plasmas (September 13 | 18, 2009, Chernogolovka, Russia). The Conference continues a tradi- tional series of meetings devoted to new theoretical and experimental results on the physics of dense non-ideal plasmas: Martzlow-Garwitz, 1980; Wus- trow, 1982; Biesenthal, 1984; Greifswald, 1986; Wustrow, 1988; Gosen, 1991; Markgrafenheide, 1993; Binz, 1995; Rostock, 1998; Greifswald, 2000; Valen- cia, 2003; Darmstadt, 2006. The following questions are covered: statistical physics and mathematical modeling (including simulation) of strongly cou- pled Coulomb systems, equilibrium properties and equation of state of dense plasmas, kinetics, transport and optical properties of dense Coulomb systems, dense hydrogen, laser and heavy-ion-produced plasmas, dense astrophysical plasmas, phase transitions in plasmas and fluids, dusty plasmas. The conference is held under financial support of the Russian Academy of Sciences, Russian Foundation for Basic Research (grant No. 09 { 02 { 06154Γ), and Dynasty Foundation. Contents 1 Statistical physics and mathematical modeling of strongly cou- pled Coulomb systems 16 1.1 Mathematical simulation of kinetic processes in the non-ideal nuclear-excited dust plasma of the noble gases Budnik A.P., Deputatova L.V., Fortov V.E., Kosarev V.A., Rykov V.A., Vladimirov V.I., JIHT RAS . 16 1.2 Diagnosics of dense plasmas via transport and optical proper- ties Reinholz H., Raitza T., R¨opke G., Wierling A., Winkel M., U. -

Micro-Cavity Fluidic Dye Lasers
Micro-Cavity Fluidic Dye Lasers M.Sc. Thesis Bjarne Helbo Student Number: c960336 Supervisors: Anders Kristensen and Aric Menon Mikroelektronik Centret (MIC) Technical University of Denmark (DTU) November 2002 Abstract i Abstract The work described in this masters thesis deals with development, fabrication, and optical characterization of micro-cavity fluidic dye lasers. The wide band fluorescence of organic dyes make them suitable as the active gain media for tunable dye lasers. Decreasing the laser cavity size down to the micron level makes dye lasers suitable for integration with existing bio/chemical microsystems. Theory for organic dyes is presented together with basic laser theory. The theory is used for explaining the behavior of the fabricated devices. Two types of micro-cavity fluidic dye laser devices were fabricated based on two dif- ferent micro-fabrication schemes. The devices were vertical emitting with fixed lasing wavelength. The initial device was a microfluidic channel defined with an KOH etch of silicon. The bottom (100) plane of the etched silicon channel was used as a deposition surface for a gold/chromium mirror. The etched surface was not smooth enough for mirror purpose and the following anodic bonding of a glass lid on the top made the surface even more rough. Due to thermal heating from the anodic bonding process the metals diffused into the silicon substrate and left the surface dull and rough, not suitable for optical mirrors. A cw argon ion laser (488 nm) was used for optical pumping of a Rhodamine 110 dye dissolved in ethanol, which was pumped through the microfluidic laser cavity. -

Chapter 2 HISTORY and DEVELOPMENT of MILITARY LASERS
History and Development of Military Lasers Chapter 2 HISTORY AND DEVELOPMENT OF MILITARY LASERS JACK B. KELLER, JR* INTRODUCTION INVENTING THE LASER MILITARIZING THE LASER SEARCHING FOR HIGH-ENERGY LASER WEAPONS SEARCHING FOR LOW-ENERGY LASER WEAPONS RETURNING TO HIGHER ENERGIES SUMMARY *Lieutenant Colonel, US Army (Retired); formerly, Foreign Science Information Officer, US Army Medical Research Detachment-Walter Reed Army Institute of Research, 7965 Dave Erwin Drive, Brooks City-Base, Texas 78235 25 Biomedical Implications of Military Laser Exposure INTRODUCTION This chapter will examine the history of the laser, Military advantage is greatest when details are con- from theory to demonstration, for its impact upon the US cealed from real or potential adversaries (eg, through military. In the field of military science, there was early classification). Classification can remain in place long recognition that lasers can be visually and cutaneously after a program is aborted, if warranted to conceal hazardous to military personnel—hazards documented technological details or pathways not obvious or easily in detail elsewhere in this volume—and that such hazards deduced but that may be relevant to future develop- must be mitigated to ensure military personnel safety ments. Thus, many details regarding developmental and mission success. At odds with this recognition was military laser systems cannot be made public; their the desire to harness the laser’s potential application to a descriptions here are necessarily vague. wide spectrum of military tasks. This chapter focuses on Once fielded, system details usually, but not always, the history and development of laser systems that, when become public. Laser systems identified here represent used, necessitate highly specialized biomedical research various evolutionary states of the art in laser technol- as described throughout this volume. -
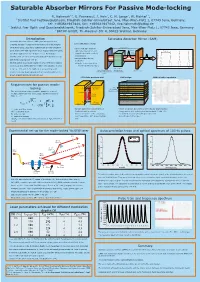
Saturable Absorber Mirrors for Passive Mode-Locking
Saturable Absorber Mirrors For Passive Mode-locking R. Hohmuth1 3, G. Paunescu2, J. Hein2, C. H. Lange3, W. Richter1 3, 1 Institut fuer Festkoerperphysik, Friedrich-Schiller-Universitaet Jena, Max-Wien-Platz 1, 07743 Jena, Germany, tel: +493641947444, fax: +493641947442, [email protected] 2 Institut fuer Optik und Quantenelektronik, Friedrich-Schiller-Universitaet Jena, Max-Wien-Platz 1, 07743 Jena, Germany 3 BATOP GmbH, Th.-Koerner-Str. 4, 99425 Weimar, Germany Introduction Saturable Absorber Mirror (SAM) Saturable absorber mirrors (SAMs) are inexpensive and schematic laser set-up compact devices for passive mode-locking of diode pumped solid state lasers. Such laser systems can provide ultrashort cavity, length L -cavity with gain medium pulse trains with high repetition rates. Typical values for pulse pulse -high reflective mirror and TRT=2L/c ) t output mirror with partially ( duration ranging from 100 fs up to 10 ps. For instance a I y t transmission i s Nd:YAG laser can be mode-locked with pulse duration of 8 ps n e -saturable absorber as t n and mean output power of 6 W. I modulator On this poster we present results for a Yb: KYW laser passive =>pulse trains spaced by mode-locked by SAMs with three different modulation depths round-trip time TRT=2L/c time t between 0.6% and 2.0%. SAMs were prepared by solid- gain medium high reflective mirror (pumped e.g. output mirror source molecular beam epitaxy with a low-temperature (LT) with saturable absorber by laser diode) (SAM) grown InGaAs absorbing quantum well. LT InGaAs quantum well SAM design SAM reflection spectrum Ta O or SiO / dielectric cover Requirements for passive mode- 25 2 conduction 71.2 nm GaAs / barrier E 7 nm c1 71.2 nm GaAs / barrier locking band Ec LT InGaAs 74.7 nm GaAs The mode-locking regime is stable agaist the onset of quantum well InGaAs 88.4 nm AlAs multiple pulsing as long as the pulse duration is smaller energy : 25x Bragg mirror than t . -

Solid State Laser
SOLID STATE LASER Edited by Amin H. Al-Khursan Solid State Laser Edited by Amin H. Al-Khursan Published by InTech Janeza Trdine 9, 51000 Rijeka, Croatia Copyright © 2012 InTech All chapters are Open Access distributed under the Creative Commons Attribution 3.0 license, which allows users to download, copy and build upon published articles even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. After this work has been published by InTech, authors have the right to republish it, in whole or part, in any publication of which they are the author, and to make other personal use of the work. Any republication, referencing or personal use of the work must explicitly identify the original source. As for readers, this license allows users to download, copy and build upon published chapters even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. Notice Statements and opinions expressed in the chapters are these of the individual contributors and not necessarily those of the editors or publisher. No responsibility is accepted for the accuracy of information contained in the published chapters. The publisher assumes no responsibility for any damage or injury to persons or property arising out of the use of any materials, instructions, methods or ideas contained in the book. Publishing Process Manager Iva Simcic Technical Editor Teodora Smiljanic Cover Designer InTech Design Team First published February, 2012 Printed in Croatia A free online edition of this book is available at www.intechopen.com Additional hard copies can be obtained from [email protected] Solid State Laser, Edited by Amin H. -

Quanta-Ray Lab-Series Pulsed Nd:YAG Lasers
Quanta-Ray Lab-Series Pulsed Nd:YAG Lasers User’s Manual 1335 Terra Bella Avenue Mountain View, CA 94043 Part Number 0000-311A, Rev. A June 2003 Preface This manual contains information you need in order to safely install, align, operate, maintain and service your Quanta-Ray Lab-Series pulsed Nd:YAG laser on a day-to-day basis. Also described is the installation and operation of the HG harmonic generator and IHS internal harmonic separator. The system comprises three main elements: the Lab-Series laser head, the power supply and a table-top controller. (The system can also be controlled remotely via the front panel RS-232 serial port.) An optional Model WA-1 heat exchanger may also be present. The “Introduction” contains a brief description of these three components and is followed by an important chapter on laser safety. The Lab-Series is a Class IV laser and, as such, emits laser radiation which can permanently damage eyes and skin, ignite fires and vaporize substances. Moreover, focused back-reflections of even a small percentage of its output energy can destroy expensive internal optical components. This section contains information about these hazards and offers suggestions on how to safe- guard against them. To minimize the risk of injury or expensive repairs, be sure to read this chapter—then carefully follow these instructions. This chapter also contains information regarding system compliance to CDRH and CE regulations. “Laser Description” contains a short section on laser theory regarding the Nd:YAG crystal rods that are used in the Lab-Series laser. Also included is a discussion of the second, third and fourth harmonic laser output gener- ated by the system.