Direct Injection of Liquid Nitrogen Into Water for a Cryogenic Engine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biogas Current Biofuels
Current Biofuels - Biogas Keywords Bioenergy, biofuel, biogas, sustainable, renewable, biomass, anaerobic, waste, bacteria, microbes, fermentation, methane. Background Biofuel feedstocks that have high water content, such as food wastes and livestock manure cannot be easily incinerated, but can produce biogas. Biogas can be burnt to produce heat for cooking, warming homes and producing electricity. It can also be compressed and used as a transport fuel in specially © istockphoto® converted vehicle engines. The digested residue is of use as fertiliser in agriculture. Biogas storage containers Biogas is 60-80% methane and is created by a process termed anaerobic digestion, leaving behind a nutrient- rich substance termed digestate. Anaerobic digestion is carried out by a range of bacteria in the absence of oxygen. A number of bacteria and yeast have been identified in biogas production. Initially carbon dioxide is produced by the decomposing organic matter until an anaerobic environment is created. After the initial digestion a group of bacteria known as methanogens convert the products into methane and carbon dioxide. Anaerobic digestion has a number of environmental benefits including production of ‘green energy and natural fertilisers. The production of biogas can substitute feedstocks for fossil fuels and artificial fertilisers, reducing the amount of greenhouse gases released into the atmosphere. The problems associated with waste disposal are also alleviated by the generation of useful products and decreased release of the potent greenhouse gas, methane, from landfill sites Biogas is successfully generated in a number of developing countries and Europe. In the UK, research is being conducted in a number of areas of biogas production including: • Assessment of how more automated production can be achieved and scaled up to make it efficient and cost e fective. -

Expanding the Use of Biogas with Fuel Cell Technologies
Expanding the Use of Biogas with Fuel Cell Technologies Biogas with Fuel Cells Workshop Sunita Satyapal National Renewable Energy Laboratory U.S. Department of Energy Golden, Colorado Fuel Cell Technologies Program Program Manager 6/11/2012 1 eere.energy.gov U.S. Energy Consumption U.S. Primary Energy Consumption by Source and Sector Renewable Electric Power Energy 8% Fuel Cells can apply to diverse Nuclear Industrial sectors Energy 9% Share of Energy Consumed Petroleum 37% by Major Sectors of the Economy, 2010 Residential & Commercial Coal 21% Residential 16% Transportation Natural Gas Electric Power 25% 29% Commercial 13% Transportation 20% Total U.S. Energy = 98 Quadrillion Btu/yr Industrial 22% Source: Energy Information Administration, Annual Energy Review 2010, Table 1.3 2 eere.energy.gov Fuel Cells – An Emerging Global Industry Fuel Cell Patents Geographic Source: Clean Distribution 2002-2011 Energy Patent Growth Index Japan 31% United States 46% Other 3% Clean Energy Patent Growth Index France 1% Korea Great Taiwan 7% Top 10 companies: GM, Honda, Samsung, Britain 1% 1% Toyota, UTC Power, Nissan, Ballard, Plug Canada Germany Power, Panasonic, Delphi Technologies 3% 7% Clean Energy Patent Growth Index[1] shows that fuel cell patents lead in the clean energy field with over 950 fuel cell patents issued in 2011. • Nearly double the second place holder, solar, which has ~540 patents. [1] http://cepgi.typepad.com/files/cepgi-4th-quarter-2011-1.pdf 3 eere.energy.gov Fuel Cells: Benefits & Market Potential The Role of Fuel Cells Key Benefits • up to 60% (electrical) Very High • up to 70% (electrical, hybrid fuel cell / Efficiency turbine) • up to 85% (with CHP) • 35–50%+ reductions for CHP systems Reduced (>80% with biogas) CO2 • 55–90% reductions for light-duty vehicles Emissions /Biogas • >95% reduction for FCEVs (vs. -

Liquid Argon
Safetygram 8 Liquid argon Liquid argon is tasteless, colorless, odorless, noncorrosive, nonflammable, and extremely cold. Belonging to the family of rare gases, argon is the most plentiful, making up approximately 1% of the earth’s atmosphere. It is monatomic and extremely inert, forming no known chemical compounds. Since argon is inert, special materials of construction are not required. However, materials of construction must be selected to withstand the low temperature of liquid argon. Vessels and piping should be designed to American Society of Mechanical Engineers (ASME) specifications or the Department of Transportation (DOT) codes for the pressures and temperatures involved. Although used more commonly in the gaseous state, argon is commonly stored and transported as a liquid, affording a more cost-effective way of providing product supply. Liquid argon is a cryogenic liquid. Cryogenic liquids are liquefied gases that have a normal boiling point below –130°F (–90°C). Liquid argon has a boiling point of –303°F (–186°C). The temperature difference between the product and the surrounding environment, even in winter, is substantial. Keeping this surrounding heat from the product requires special equipment to store and handle cryogenic liquids. A typical system consists of the following components: a cryogenic storage tank, one or more vaporizers, a pressure control system, and all of the piping required for fill, vaporization. The cryogenic tank is constructed, in principle, like a vacuum bottle. It is designed to keep heat away from the liquid that is contained in the inner vessel. Vaporizers convert the liquid argon to its gaseous state. A pressure control manifold controls the pressure at which the gas is fed to the process. -

WHY BIOGAS? Biogas Systems Protect Our Air, Water and Soil While Recycling Organic Material to Produce Renewable Energy and Soil Products
WHY BIOGAS? Biogas systems protect our air, water and soil while recycling organic material to produce renewable energy and soil products. In cities, biogas systems recycle food scraps and wastewater sludge, reducing municipal costs and avoiding transport to disposal sites. In rural areas, biogas systems make agriculture more sustainable and create additional revenue streams for farmers. Since biogas systems prevent greenhouse gases, like methane, from entering the atmosphere, all biogas systems make our air cleaner to breathe and combat climate change, displacing fossil fuels. Biogas systems produce soil products that recycle nutrients, contributing to healthier soils 1211 Connecticut Avenue NW, Suite 650 and creating opportunities to eliminate nutrient runoff that pollutes our waterways. Waste management, renewable Washington, DC 20036-2701 energy and fuels, clean air, healthy soils and crystal clear waterways—you can get all of this when you build a new 202-640-6595 biogas system. [email protected] Use the interactive map at https://americanbiogascouncil.org/resources/biogas-projects/ Operational U.S. Biogas Systems The U.S. has over 2,200 sites producing biogas in all 50 states: 253 anaerobic digesters on farms, 1,269 water resource recovery facilities utilizing anaerobic 101 digesters, 68 stand-alone systems that digest food waste, and 652 landfill gas projects. For comparison, Europe has over Alaska 10,000 operating digesters, with some communities essentially fossil fuel free because of these systems. In 2018, investment in new biogas systems Puerto Rico totaled $1 billion. Over the last five years, total investment in the U.S. biogas industry has been growing at an annual rate of 12%. -

Fuel Properties Comparison
Alternative Fuels Data Center Fuel Properties Comparison Compressed Liquefied Low Sulfur Gasoline/E10 Biodiesel Propane (LPG) Natural Gas Natural Gas Ethanol/E100 Methanol Hydrogen Electricity Diesel (CNG) (LNG) Chemical C4 to C12 and C8 to C25 Methyl esters of C3H8 (majority) CH4 (majority), CH4 same as CNG CH3CH2OH CH3OH H2 N/A Structure [1] Ethanol ≤ to C12 to C22 fatty acids and C4H10 C2H6 and inert with inert gasses 10% (minority) gases <0.5% (a) Fuel Material Crude Oil Crude Oil Fats and oils from A by-product of Underground Underground Corn, grains, or Natural gas, coal, Natural gas, Natural gas, coal, (feedstocks) sources such as petroleum reserves and reserves and agricultural waste or woody biomass methanol, and nuclear, wind, soybeans, waste refining or renewable renewable (cellulose) electrolysis of hydro, solar, and cooking oil, animal natural gas biogas biogas water small percentages fats, and rapeseed processing of geothermal and biomass Gasoline or 1 gal = 1.00 1 gal = 1.12 B100 1 gal = 0.74 GGE 1 lb. = 0.18 GGE 1 lb. = 0.19 GGE 1 gal = 0.67 GGE 1 gal = 0.50 GGE 1 lb. = 0.45 1 kWh = 0.030 Diesel Gallon GGE GGE 1 gal = 1.05 GGE 1 gal = 0.66 DGE 1 lb. = 0.16 DGE 1 lb. = 0.17 DGE 1 gal = 0.59 DGE 1 gal = 0.45 DGE GGE GGE Equivalent 1 gal = 0.88 1 gal = 1.00 1 gal = 0.93 DGE 1 lb. = 0.40 1 kWh = 0.027 (GGE or DGE) DGE DGE B20 DGE DGE 1 gal = 1.11 GGE 1 kg = 1 GGE 1 gal = 0.99 DGE 1 kg = 0.9 DGE Energy 1 gallon of 1 gallon of 1 gallon of B100 1 gallon of 5.66 lb., or 5.37 lb. -

Compressed Liquid Gases (Cryogens) Laboratory Safe Work Practices
Compressed Liquid Gases (Cryogens) Laboratory Safe Work Practices Source: Section 3.9 of Guidelines for Safe Work Practices in Human and Animal Medical Diagnostic Laboratories: Recommendations of a CDC-convened, Biosafety Blue Ribbon Panel; excerpted by Vanderbilt OCRS, 10.2020. Compressed Liquid Gases (Cryogens) Cryogenic liquids are liquefied gases that have a normal boiling point below -238°F (-150°C). Liquid nitrogen is used in the microbiology laboratory to freeze and preserve cells and virus stocks. The electron microscopy laboratory, frozen section suites, and grossing stations for surgical pathology frequently use liquid nitrogen; some laboratories also use liquid helium. The principal hazards associated with handling cryogenic fluids include cold contact burns and freezing, asphyxiation, explosion and material embrittlement. Cold contact burns and freezing • Liquid nitrogen is dangerously cold (-320°F [-196°C]), and • Wear loose-fitting thermal gloves with elbow-length cuffs skin contact with either the liquid or gas phase can when filling dewars. Ensure that gloves are loose enough to immediately cause frostbite. At -450°F (-268°C), liquid be thrown off quickly if they contact the liquid. helium is dangerous and cold enough to solidify atmospheric • Never place gloved hands into liquid nitrogen or into the air. liquid nitrogen stream when filling dewars. Gloves are not • Always wear eye protection (face shield over safety goggles). rated for this type of exposure. Insulated gloves are The eyes are extremely sensitive to freezing, and liquid designed to provide short-term protection during handling of nitrogen or liquid nitrogen vapors can cause eye damage. hoses or dispensers and during incidental contact with the • Do not allow any unprotected skin to contact uninsulated liquid. -

Cryogenics – the Basics Or Pre-Basics Lesson 1 D
Cryogenics – The Basics or Pre-Basics Lesson 1 D. Kashy Version 3 Lesson 1 - Objectives • Look at common liquids and gases to get a feeling for their properties • Look at Nitrogen and Helium • Discuss Pressure and Temperature Scales • Learn more about different phases of these fluids • Become familiar with some cryogenic fluids properties Liquids – Water (a good reference) H2O density is 1 g/cc 10cm Total weight 1000g or 1kg (2.2lbs) Cube of water – volume 1000cc = 1 liter Liquids – Motor Oil 10cm 15W30 density is 0.9 g/cc Total weight 900g or 0.9 kg (2lbs) Cube of motor oil – volume 1000cc = 1 liter Density can and usually does change with temperature 15W30 Oil Properties Density Curve Density scale Viscosity scale Viscosity Curve Water density vs temperature What happens here? What happens here? Note: This plot is for SATURATED Water – Discussed soon Water and Ice Water Phase Diagram Temperature and Pressure scales • Fahrenheit: 32F water freezes 212 water boils (at atmospheric pressure) • Celsius: 0C water freezes and 100C water boils (again at atmospheric pressure) • Kelvin: 273.15 water freezes and 373.15 water boils (0K is absolute zero – All motion would stop even electrons around a nucleus) • psi (pounds per square in) one can reference absolute pressure or “gage” pressure (psia or psig) • 14.7psia is one Atmosphere • 0 Atmosphere is absolute vacuum, and 0psia and -14.7psig • Standard Temperature and Pressure (STP) is 20C (68F) and 1 atm Temperature Scales Gases– Air Air density is 1.2kg/m3 => NO Kidding! 100cm =1m Total weight -

Cryogenic Liquid Nitrogen Vehicles (ZEV's)
International Journal of Scientific and Research Publications, Volume 6, Issue 9, September 2016 562 ISSN 2250-3153 Cryogenic Liquid Nitrogen Vehicles (ZEV’S) K J Yogesh Department Of Mechanical Engineering, Jain Engineering College, Belagavi Abstract- As a result of widely increasing air pollution available zero emission vehicle (ZEV) meeting it's standards are throughout the world & vehicle emissions having a major the electrically recharged ones, however these vehicles are also contribution towards the same, it makes its very essential to not a great success in the society due to its own limitations like engineer or design an alternative to the present traditional initial cost, slow recharge, speeds etc. Lead acid & Ni-Cd gasoline vehicles. Liquid nitrogen fueled vehicles can act as an batteries are the past of major technologies in the electric excellent alternative for the same. Liquefied N2 at cryogenic vehicles. They exhibit specific energy in the range of 30-40 W- temperatures can replace conventional fuels in cryogenic heat hr/kg. Lead- acid batteries take hours to recharge & the major engines used as a propellant. The ambient temperature of the drawback of the batteries in all the cases is their replacement surrounding vaporizes the liquid form of N2 under pressure & periodically. This directly/indirectly increases the operating cost leads to the formation of compressed N2 gas. This gas actuates a when studied carefully & thereby not 100% acceptable. pneumatic motor. A combination of multiple reheat open Recent studies make it clear that the vehicles using liquid Rankine cycle & closed Brayton cycle are involved in the nitrogen as their means provide an excellent alternative before process to make use of liquid N2 as a non-polluting fuel. -

Final Report Study on the Potential of Increased Use of LPG for Cooking in Developing Countries
Final Report Study on the Potential of Increased Use of LPG for Cooking in Developing Countries September 2020 TABLE OF CONTENTS Executive Summary ....................................................................................................................................................................... 2 List of Abbreviations ...................................................................................................................................................................... 6 Preface .......................................................................................................................................................................................... 7 1 Introduction.......................................................................................................................................................................... 8 1.1 General ................................................................................................................................................................................. 8 1.2 Background ........................................................................................................................................................................... 8 2 Purpose and Scope of the Study ............................................................................................................................................ 9 2.1 Purpose of the Study ........................................................................................................................................................... -

Process Technologies and Projects for Biolpg
energies Review Process Technologies and Projects for BioLPG Eric Johnson Atlantic Consulting, 8136 Gattikon, Switzerland; [email protected]; Tel.: +41-44-772-1079 Received: 8 December 2018; Accepted: 9 January 2019; Published: 15 January 2019 Abstract: Liquified petroleum gas (LPG)—currently consumed at some 300 million tonnes per year—consists of propane, butane, or a mixture of the two. Most of the world’s LPG is fossil, but recently, BioLPG has been commercialized as well. This paper reviews all possible synthesis routes to BioLPG: conventional chemical processes, biological processes, advanced chemical processes, and other. Processes are described, and projects are documented as of early 2018. The paper was compiled through an extensive literature review and a series of interviews with participants and stakeholders. Only one process is already commercial: hydrotreatment of bio-oils. Another, fermentation of sugars, has reached demonstration scale. The process with the largest potential for volume is gaseous conversion and synthesis of two feedstocks, cellulosics or organic wastes. In most cases, BioLPG is produced as a byproduct, i.e., a minor output of a multi-product process. BioLPG’s proportion of output varies according to detailed process design: for example, the advanced chemical processes can produce BioLPG at anywhere from 0–10% of output. All these processes and projects will be of interest to researchers, developers and LPG producers/marketers. Keywords: Liquified petroleum gas (LPG); BioLPG; biofuels; process technologies; alternative fuels 1. Introduction Liquified petroleum gas (LPG) is a major fuel for heating and transport, with a current global market of around 300 million tonnes per year. -

Capacity Enhancement of Indigenous Expansion Engine Based Helium Liquefier
IOP Conference Series: Materials Science and Engineering PAPER • OPEN ACCESS Capacity enhancement of indigenous expansion engine based helium liquefier To cite this article: R S Doohan et al 2017 IOP Conf. Ser.: Mater. Sci. Eng. 171 012011 View the article online for updates and enhancements. This content was downloaded from IP address 170.106.202.8 on 27/09/2021 at 00:16 ICECICMC IOP Publishing IOP Conf. Series: Materials Science and Engineering 171 (2017) 012011 doi:10.1088/1757-899X/171/1/012011 International Conference on Recent Trends in Physics 2016 (ICRTP2016) IOP Publishing Journal of Physics: Conference Series 755 (2016) 011001 doi:10.1088/1742-6596/755/1/011001 Capacity enhancement of indigenous expansion engine based helium liquefier R S Doohan1, P K Kush1, G Maheshwari2 1Raja Ramanna Centre for Advanced Technology, Indore, Madhya Pradesh India. 2Institute of Engineering and Technology, DAVV, Indore, Madhya Pradesh, India. E-mail: [email protected] Abstract. Development of technology and understanding for large capacity helium refrigeration and liquefaction at helium temperature is indispensable for coming-up projects. A new version of helium liquefier designed and built to provide approximately 35 liters of liquid helium per hour. The refrigeration capacity of this reciprocating type expansion engine machine has been increased from its predecessor version with continuous improvement and deficiency debugging. The helium liquefier has been built using components by local industries including cryogenic Aluminum plate fin heat exchangers. Two compressors with nearly identical capacity have been deployed for the operation of system. Together they consume about 110 kW of electric power. The system employs liquid Nitrogen precooling to enhance liquid Helium yield. -
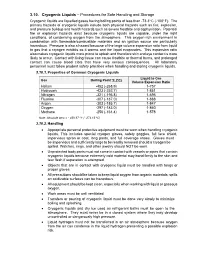
3.10. Cryogenic Liquids – Procedures for Safe Handling and Storage Cryogenic Liquids Are Liquefied Gases Having Boiling Points of Less Than -73.3O C (-100O F)
3.10. Cryogenic Liquids – Procedures for Safe Handling and Storage Cryogenic liquids are liquefied gases having boiling points of less than -73.3o C (-100o F). The primary hazards of cryogenic liquids include both physical hazards such as fire, explosion, and pressure buildup and health hazards such as severe frostbite and asphyxiation. Potential fire or explosion hazards exist because cryogenic liquids are capable, under the right conditions, of condensing oxygen from the atmosphere. This oxygen-rich environment in combination with flammable/combustible materials and an ignition source are particularly hazardous. Pressure is also a hazard because of the large volume expansion ratio from liquid to gas that a cryogen exhibits as it warms and the liquid evaporates. This expansion ratio also makes cryogenic liquids more prone to splash and therefore skin and eye contact is more likely to occur. Contact with living tissue can cause frostbite or thermal burns, and prolonged contact can cause blood clots that have very serious consequences. All laboratory personnel must follow prudent safety practices when handling and storing cryogenic liquids. 3.10.1. Properties of Common Cryogenic Liquids Liquid to Gas Gas Boiling Point oF (oC) Volume Expansion Ratio Helium -452 (-268.9) 1-757 Hydrogen -423 (-252.7) 1-851 Nitrogen -321 (-195.8) 1-696 Fluorine -307 (-187.0) 1-888 Argon -303 (-185.7) 1-847 Oxygen -297 (-183.0) 1-860 Methane -256 (-161.4) 1-578 Note: Absolute zero = - 459.67 oF (- 273.15 oC) 3.10.2. Handling Appropriate personal protective equipment must be worn when handling cryogenic liquids.