Puddling: a New Look at an Old Process
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Two Steps Forward, One Step Back: a Brief History of Corporate Citizenship and Corporate Social Responsibility
Two Steps Forward, One Step Back: A Brief History of Corporate Citizenship and Corporate Social Responsibility Stephen Jordan with B.J. Parker BCLC’s mission is to promote better business and society relations and improve long-term social and economic conditions by: • Communicating the U.S. private sector’s unique and valuable contributions • Cultivating strategies and practices that achieve positive results • Coordinating public-private partnerships and coalitions 2 TABLE OF CONTENTS CHAPTER ONE: DEFINING CORPORATE CITIZENSHIP AND CORPORATE SOCIAL RESPONSIBILITY . .1 CHAPTER TWO: THE ORIGINS OF CAPITALISM AND CORPORATE CITIZENSHIP . .6 CHAPTER THREE: THE RISE OF THE INDUSTRIAL REVOLUTION AND PATERNALISM . 9 CHAPTER FOUR: THE GILDED AGE AND PERSONAL PHILANTHROPY . .12 CHAPTER FIVE: THE FIRST ETHICAL CORPORATIONS . 15 CHAPTER SIX: THE COMMUNITY CHEST MOVEMENT, THE GREAT DEPRESSION, AND WORLD WAR II . 20 CHAPTER SEVEN: THE 1950s - 1970s . .26 CHAPTER EIGHT: 1980 AND THE DAWN OF THE MODERN CORPORATE CITIZENSHIP MOVEMENT . 31 CHAPTER NINE: THE VIEW FROM OUTSIDE . .35 CHAPTER TEN: THE CONTINUING EVOLUTION OF CORPORATE CITIZENSHIP . 41 CHAPTER ELEVEN: DIFFERENT WAYS THE HISTORY OF CORPORATE CITIZENSHIP COULD BE TOLD . 46 Cover photos (clockwise): Andrew Carnegie, Henry Ford, Indra Nooyi (courtesy of PepsiCo), Thomas J. Watson (courtesy of IBM), Robert Wood Johnson II, Bill and Melinda Gates (Wikipedia Commons User Kjetil Ree). About the Authors Stephen Jordan is the founder and executive director of the Business Civic Leadership Center (BCLC), the corporate citizenship affiliate of the U.S. Chamber of Commerce. He is married and has two children. B.J. Parker is a professional writer and editor with more than 10 years’ experience writing business education materials for leading educational publishers. -

National Register of Historic Places Multiple Property
NFS Form 10-900-b 0MB No. 1024-0018 (Jan. 1987) United States Department of the Interior National Park Service National Register of Historic Places Multipler Propertyr ' Documentation Form NATIONAL This form is for use in documenting multiple property groups relating to one or several historic contexts. See instructions in Guidelines for Completing National Register Forms (National Register Bulletin 16). Complete each item by marking "x" in the appropriate box or by entering the requested information. For additional space use continuation sheets (Form 10-900-a). Type all entries. A. Name of Multiple Property Listing ____Iron and Steel Resources of Pennsylvania, 1716-1945_______________ B. Associated Historic Contexts_____________________________ ~ ___Pennsylvania Iron and Steel Industry. 1716-1945_________________ C. Geographical Data Commonwealth of Pennsylvania continuation sheet D. Certification As the designated authority under the National Historic Preservation Act of 1966, as amended, J hereby certify that this documentation form meets the National Register documentation standards and sets forth requirements for the listing of related properties consistent with the National Register criteria. This submission meets the procedural and professional requiremerytS\set forth iri36JCFR PafrfsBOfcyid the Secretary of the Interior's Standards for Planning and Evaluation. Signature of certifying official Date / Brent D. Glass Pennsylvania Historical & Museum Commission State or Federal agency and bureau I, hereby, certify that this multiple -

Comparative Properties of Wrought Iron Made by Hand Puddling and by the Aston Process
RP124 COMPARATIVE PROPERTIES OF WROUGHT IRON MADE BY HAND PUDDLING AND BY THE ASTON PROCESS By Henry S. Rawdon and 0. A. Knight ABSTRACT The hand-puddling method of making wrought iron has not greatly changed for a century. More economical methods in the manufacture of thjs product is the crying need of the industry. A radically new process, recently developed, is now coming into commercial use, in which pig iron, which h>as been refined in a Bessemer converter, is poured into molten slag so as to produce intimate mingling of the two. A comparison of the properties of wrought iron made thus with that made by hand puddling forms the subject of this report. The test results failed to show any marked difference in the products of the two processes. The new product appears to have all of the essential properties usually connoted by the name—wrought iron, CONTENTS Page I. Introduction 954 1. Resume of the Aston process 955 II. Purpose and scope of the investigation 959 III. Materials and methods 960 1. Materials 960 (a) Pipe 960 (6) "Rounds" 961 (c) Slag 962 2. Methods 962 IV. Results 962 1. Composition 962 2. Density 964 3. Mechanical properties 965 (a) Pipe materials 965 (1) Tensile properties 965 (2) Torsional properties 970 (3) Flattening tests 971 (6) 1-inch rounds 972 (1) Tensile properties 972 (2) Torsional properties 973 (3) Impact resistance 973 4. Corrosion resistance 976 (a) Laboratory corrosion tests 976 (6) Electrolytic solution potential 979 5. Structural examination 979 (a) Pipe materials 980 (1) BaU 980 (2) Muck bar 980 (3) Skelp 981 (4) Pipe 981 (&) 1-inch rounds 981 (c) Slag 981 953 : 954 Bureau of Standards Journal of Research [vol. -

Final Exam Questions Generated by the Class
Final Exam Questions Generated by the Class Module 8 – Iron and Steel Describe some of the business practices that Carnegie employed that allowed him to take command of the steel industry. Hard driving, vertical integration, price making Which of the following was/is NOT a method used to make steel? A. Puddling B. Bessemer process C. Basic oxygen process D. Arc melting E. None of the above What are the three forms of iron, and what is the associated carbon content of each? Wrought <.2% Steel .2-2.3% Cast Iron 2.3-4.2% How did Andrew Carnegie use vertical integration to gain control of the steel market? Controlled the entire steel making process from mining to final product Who created the best steel for several hundred years while making swords during the 1500’s? A. Syria B. Egypt C. Japan D. England Describe the difference between forging and casting. When forging, you beat and hammer the material into the desired shape. When casting, you pour liquid into a mold to shape it. Describe the difference between steel and wrought iron. Steel has less carbon Which of the following forms of iron has a low melting point and is not forgeable? A. Steel B. Pig Iron C. Wrought Iron D. None of the Above What two developments ushered in the transition from the Bronze Age to the Iron Age? More iron ore and greater ability to change its properties using readily available alloying agent (carbon) 1 Final Exam Questions Generated by the Class What is the difference between ferrite and austenite? A. -

The Rate and Direction of Inventive Activity Revisited
This PDF is a selection from a published volume from the National Bureau of Economic Research Volume Title: The Rate and Direction of Inventive Activity Revisited Volume Author/Editor: Josh Lerner and Scott Stern, editors Volume Publisher: University of Chicago Press Volume ISBN: 0-226-47303-1; 978-0-226-47303-1 (cloth) Volume URL: http://www.nber.org/books/lern11-1 Conference Date: September 30 - October 2, 2010 Publication Date: March 2012 Chapter Title: The Rate and Direction of Invention in the British Industrial Revolution: Incentives and Institutions Chapter Authors: Ralf R. Meisenzahl, Joel Mokyr Chapter URL: http://www.nber.org/chapters/c12364 Chapter pages in book: (p. 443 - 479) 9 The Rate and Direction of Invention in the British Industrial Revolution Incentives and Institutions Ralf R. Meisenzahl and Joel Mokyr 9.1 Introduction The Industrial Revolution was the fi rst period in which technological progress and innovation became major factors in economic growth. There is by now general agreement that during the seventy years or so traditionally associated with the Industrial Revolution, there was little economic growth as traditionally measured in Britain, but that in large part this was to be expected.1 The sectors in which technological progress occurred grew at a rapid rate, but they were small in 1760, and thus their effect on growth was limited at fi rst (Mokyr 1998, 12– 14). Yet progress took place in a wide range of industries and activities, not just in cotton and steam. A full description of the range of activities in which innovation took place or was at least attempted cannot be provided here, but inventions in some pivotal industries such as iron and mechanical engineering had backward linkages in many more traditional industries. -
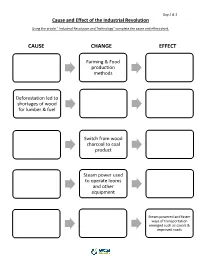
Cause Change Effect
Day 2 & 3 Cause and Effect of the Industrial Revolution Using the article “ Industrial Revolution and Technology” complete the cause and effect chart. CAUSE CHANGE EFFECT Farming & Food production methods Deforestation led to shortages of wood for lumber & fuel Switch from wood charcoal to coal product Steam power used to operate looms and other equipment Steam powered and faster ways of transportation emerged such as canals & improved roads Day 3 & 4 Industrial Revolution Timeline 1721: Cromford Mill was the first factory with the first water powered cotton spinning mill. 1733: Flying Shuttle made weaving faster, invented by John Kay. 1760: Industrial Revolution begins in England 1767: Spinning Jenny, a machine that can spin thread more quickly was invented James 1769: Water Frame, improved the Spinning Hargreaves. Jenny, ran on water instead of human labor. 1783: Henry Cort patented the puddling process which allowed wrought iron to be produced in large quantities. 1784: Steam Powered Loom that turned thread into cloth is invented by Edmund Cartwright. 1792-3: Eli Whitney invents the cotton gin, changing the textile sector. Now cotton fiber was separated from seeds through machine. 1831: Electric Generator led to improvements to create the first generator to support a factory. Invented by Michael Faraday. 1837: Telegraph made transmission of messages easier. Invented by William Fothergill & Charles Wheatstone. 1856: Bessemer Converter was the first process of manufacturing steel that wasn’t expensive. Day 3 & 4 Sources Brodsky Schur, J. (2016, September 23). Eli Whitney's Patent for the Cotton Gin. Retrieved June 22, 2021, from http://www.archives.gov/education/lessons/cotton-gin-patent Karpiel, F., Krull, K., & Wiggins, G. -

Ironworks and Iron Monuments Forges Et
IRONWORKS AND IRON MONUMENTS FORGES ET MONUMENTS EN FER I( ICCROM i ~ IRONWORKS AND IRON MONUMENTS study, conservation and adaptive use etude, conservation et reutilisation de FORGES ET MONUMENTS EN FER Symposium lronbridge, 23-25 • X •1984 ICCROM rome 1985 Editing: Cynthia Rockwell 'Monica Garcia Layout: Azar Soheil Jokilehto Organization and coordination: Giorgio Torraca Daniela Ferragni Jef Malliet © ICCROM 1985 Via di San Michele 13 00153 Rome RM, Italy Printed in Italy Sintesi Informazione S.r.l. CONTENTS page Introduction CROSSLEY David W. The conservation of monuments connected with the iron and steel industry in the Sheffield region. 1 PETRIE Angus J. The No.1 Smithery, Chatham Dockyard, 1805-1984 : 'Let your eye be your guide and your money the last thing you part with'. 15 BJORKENSTAM Nils The Swedish iron industry and its industrial heritage. 37 MAGNUSSON Gert The medieval blast furnace at Lapphyttan. 51 NISSER Marie Documentation and preservation of Swedish historic ironworks. 67 HAMON Francoise Les monuments historiques et la politique de protection des anciennes forges. 89 BELHOSTE Jean Francois L'inventaire des forges francaises et ses applications. 95 LECHERBONNIER Yannick Les forges de Basse Normandie : Conservation et reutilisation. A propos de deux exemples. 111 RIGNAULT Bernard Forges et hauts fourneaux en Bourgogne du Nord : un patrimoine au service de l'identite regionale. 123 LAMY Yvon Approche ethnologique et technologique d'un site siderurgique : La forge de Savignac-Ledrier (Dordogne). 149 BALL Norman R. A Canadian perspective on archives and industrial archaeology. 169 DE VRIES Dirk J. Iron making in the Netherlands. 177 iii page FERRAGNI Daniela, MALLIET Jef, TORRACA Giorgio The blast furnaces of Capalbio and Canino in the Italian Maremma. -

History of Metallurgy
History of Metallurgy by Rochelle Forrester Copyright © 2019 Rochelle Forrester All Rights Reserved The moral right of the author has been asserted Anyone may reproduce all or any part of this paper without the permission of the author so long as a full acknowledgement of the source of the reproduced material is made. Second Edition Published 30 September 2019 Preface This paper was written in order to examine the order of discovery of significant developments in the history of metallurgy. It is part of my efforts to put the study of social and cultural history and social change on a scientific basis capable of rational analysis and understanding. This has resulted in a hard copy book How Change Happens: A Theory of Philosophy of History, Social Change and Cultural Evolution and a website How Change Happens Rochelle Forrester’s Social Change, Cultural Evolution and Philosophy of History website. There are also philosophy of history papers such as The Course of History, The Scientific Study of History, Guttman Scale Analysis and its use to explain Cultural Evolution and Social Change and Philosophy of History and papers on Academia.edu, Figshare, Humanities Commons, Mendeley, Open Science Framework, Orcid, Phil Papers, SocArXiv, Social Science Research Network, Vixra and Zenodo websites. This paper is part of a series on the History of Science and Technology. Other papers in the series are The Invention of Stone Tools Fire The Neolithic Revolution The Invention of Pottery History -

The Iron Industry Energy Transition
Energy Policy 50 (2012) 24–34 Contents lists available at SciVerse ScienceDirect Energy Policy journal homepage: www.elsevier.com/locate/enpol The iron industry energy transition Nuno Luis Madureira n,1 University, ISCTE-IUL CEHC, no. 321 B, Av. Forc-as Armadas, 1600 Lisbon, Portugal article info abstract Article history: This article examines the energy transition in the iron industry and studies the consequence of this Received 8 October 2011 switch to coal-fueling technology upon forests: what happens to long-lived energy carriers when a new Accepted 1 March 2012 source of heat and power makes significant inroads into their own markets? What factors underpin the Available online 30 March 2012 substitution of older raw materials by new ones? The major lesson to be drawn from the iron industry Keywords: energy transition points to the fact that within the ‘‘transitional’’ time-frame one may expect either the Iron industry effective substitution of the older energy carrier or incentives to its actual expansion. Forests & 2012 Elsevier Ltd. All rights reserved. Fuel efficiency 1. Introduction biofuels to coal’’ (Smil, 2010, p. 28). We know, furthermore, that Netherlands with its abundant peat and Portugal with its minor In its broader sense, ‘‘energy transition’’ describes a structural coal consumption levels constitute exceptions to the classical change in the relationship between men and natural resources. It scheme of energy transition (Gales et al., 2007; Henriques, 2009). was mostly in the nineteenth century that mankind system- Much more is certainly to be learned from applied research in this atically began to tap into the legacy of carbon and carbon and area in the forthcoming years. -

An Industrial Revolution in Iron—Technology, Organisation and Markets, 1760–1870
CHAPTER FOUR AN INDUSTRIAL REVOLUTION IN IRON—TECHNOLOGY, ORGANISATION AND MARKETS, 1760–1870 Baltic iron had come to dominate the British market because of the incapacity of Britain’s own forge sector. British ironmasters lacked the energy resources to keep pace with the heightening demand for mal- leable iron on their domestic market. Some ironmasters sought to over- come this de ciency by organisational means. They hoped to raid the abundant energy reserves of British North America by transferring the preliminary stages of the production chain to the colonies. An Atlantic iron trade, with smelting out-sourced to the charcoal-rich plantations, would be a reproof to the ‘Ignorance & wrong reasonings’ of those Swedish ministers who maintained ‘that England can not be without their Iron’.1 That hope was, as we have seen, thwarted. The alternative to organisational re-jigging was technological transfor- mation. Technological revolution there was, as every textbook on British economic history makes clear. Smelting with coke and the development of coal- red re\ ning methods, most notably Henry Cort’s puddling technique, freed the British iron industry from its dependence upon vegetable fuel in spectacular fashion. Yet technological change could not be conjured up at will. The development of effective coal-based tech- nologies was a drawn-out, tortuous business. Some elements of the ‘coal technology package’ were present by the rst decade of the eighteenth century, but it was not until the 1790s that the iron industry turned fully to a mineral fuel platform. Indeed, it was not until the Napoleonic era that the combination of coke smelting, puddling furnaces, rolling mills, and steam power became the industry standard. -

The Contributions of Warfare with Revolutionary and Napoleonic France to the Consolidation and Progress of the British Industrial Revolution
CORE Metadata, citation and similar papers at core.ac.uk Provided by LSE Research Online Economic History Working Papers No: 264/2017 The Contributions of Warfare with Revolutionary and Napoleonic France to the Consolidation and Progress of the British Industrial Revolution Revised Version of Working Paper 150 Patrick O’Brien London School of Economics St Anthony’s College, Oxford Economic History Department, London School of Economics and Political Science, Houghton Street, London, WC2A 2AE, London, UK. T: +44 (0) 20 7955 7084. F: +44 (0) 20 7955 7730 LONDON SCHOOL OF ECONOMICS AND POLITICAL SCIENCE DEPARTMENT OF ECONOMIC HISTORY WORKING PAPERS NO. 264 – JUNE 2017 The Contributions of Warfare with Revolutionary and Napoleonic France to the Consolidation and Progress of the British Industrial Revolution Patrick Karl O’Brien London School of Economics St Anthony’s College, Oxford “Great Britain is under weightier obligation to no mortal man than to this very villain. For whereby the occurrences whereof he is the author, her greatness prosperity, and wealth, have attained their present elevation.” A Prussian General’s reference to Napoleon at the Congress of Vienna, 18151 Abstract This revised and reconfigured essay surveys a range of printed secondary sources going back to publications of the day (as well as includes research in primary sources) in order to revive a traditional and unresolved debate on economic connexions between the French and Industrial Revolutions. It argues that the costs flowing from the reallocation of labour, capital -

Wrought Iron Manufacture
19th Century Wrought Iron Manufacture in Germany Photographs of Models Displayed in the Deutsche Technical Museum in Munich These splendid models are displayed in a series of large cabinets which I saw on a visit around 2012. As you will see everything looks extremely life-like, and by photographing the models from different positions and enlarging portions of the pictures, one gets the appearance of movement. I was most interested in the manufacture of wrought iron by the “puddling” technique. Here about 100-200 kg of pig iron is melted down in a small furnace, in contact with iron oxides and the excess oxygen in the combustion gases. The main effect is to oxidise the carbon in the pig iron. Accordingly, the melting temperature of the mass of material rises, until the whole lot becomes semi solid at around 1400-1500°C. One reason for this peculiar state is that the mass also contains semi-molten silicate based slag, this being derived from the combination of the silicon in the pig iron with iron oxides. The mass is carved up, while in the furnace into “balls”, which are separately removed and then, while still at around 1500°C, the slag is squeezed out underneath a forging hammer. Although there are many cross sections shown on the internet of the wrought iron “puddling” furnaces, as they were called, the one below is the only decent photograph, I know, showing an external view. It is of the iron works in Portrack, Stockton on Tees, known locally as the “Malleable”. My great grandfather was a puddler.