Complications of Colonoscopy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rectal Foreign Body: a Primer for Emergency Physicians Bobby Desai
Desai International Journal of Emergency Medicine 2011, 4:73 http://www.intjem.com/content/4/1/73 CASEREPORT Open Access Visual diagnosis: Rectal foreign body: A primer for emergency physicians Bobby Desai Abstract We present a case that is occasionally seen within emergency departments, namely a rectal foreign body. After presentation of the case, a discussion concerning this entity is given, with practical information on necessity of an accurate and thorough history and removal of the object for clinicians. Case anoscope inserted. The object could not be visualized, A 39-year-old male presented to the Emergency Depart- and therefore no attempt was made to remove it. Gen- ment with vague complaints of abdominal pain and con- eral surgery was consulted to see the patient and stipation. He stated that the abdominal pain was dull decided to take him to the operating room for removal. and crampy in nature and generalized in distribution. The patient agreed to this. Furthermore, he stated that he had not had a bowel The object was noted to be the extension arm of a movement in 2 days, though he felt as if he had to have vacuum cleaner. It was removed according to notes with one. He denied constitutional complaints of fevers, some difficulty and the patient was admitted to the hos- chills, nausea, and vomiting, and denied urinary com- pital for observation and intravenous antibiotics. The plaints as well. patient was subsequently discharged 2 days later in The patient’s vital signs were: temperature 37.2°C, excellent condition. Upon social work discharge, he was pulse 87 beats per minute, respiratory rate of 20 per again asked how that apparatus managed to be placed minute, and blood pressure 130/84 mmHg. -

Perforation Due to a Rectal Foreign Body and Radiological Findings
Case Report Ann Colorectal Res 2021;9(1):44-46. Perforation Due to a Rectal Foreign Body and Radiological Findings Saim Turkoglu1, MD; Adem Yokuş2, MD; Fırat Aslan3, MD 1Van Traınıng and Research Hospıtal, Van, Turkey 2Department of Radiology, Medical Faculty, Yuzuncu Yıl University, Van, Turkey 3Department of General Surgery, Medical Faculty, Yuzuncu Yıl University, Van, Turkey *Corresponding authors: Received: 01-03-2021 Saim Turkoglu, Van Traınıng and Research Hospıtal, Van,Turkey Revised: 03-04-2021 Tel: +90 5356452865; Fax: +90 432 2150471 Accepted: 05-04-2021 Email: [email protected] Abstract Introduction: Rectal foreign bodies have been increasingly seen and cause urgent surgical complications. Diagnosis and treatment of these cases in emergency departments may be difficult. The effective use of radiological imaging techniques can accelerate and facilitate this process. Case Presentation: A 65-year-old male patient, who underwent computed tomography (CT) with the suspicion of a foreign body in the rectum, was admitted to the emergency outpatient clinic. The patient was a male with a psychiatric illness who later underwent emergency surgery. Since the patient had impaired consciousness during the examination, anamnesis could not be obtained, so the initial impression upon surgical consultation was perforation due to rectal tumoral thickening. In almost all cases, plain radiography is sufficient and can eliminate diagnostic difficulties. However, this is not possible for non-opaque objects. Therefore, the CT scan played an important role in the diagnosis of this patient. A 30 cm foreign body, identified as salami, was removed from the abdomen of the patient, who was later taken for emergency surgery. -

Surgical Management of Anorectal Foreign Bodies H Cinar, M Berkesoglu1, M Derebey2, E Karadeniz3, C Yildirim4, K Karabulut2, T Kesicioglu5, K Erzurumlu2
[Downloaded free from http://www.njcponline.com on Monday, June 11, 2018, IP: 102.251.47.77] Original Article Surgical Management of Anorectal Foreign Bodies H Cinar, M Berkesoglu1, M Derebey2, E Karadeniz3, C Yildirim4, K Karabulut2, T Kesicioglu5, K Erzurumlu2 Department of General Purpose: Anorectal foreign bodies (AFBs) inserted into anus constitute one of Surgery, Faculty of Medicine, the most important problems needing surgical emergency due to its complications. Ordu University, Ordu, 1Department of General We describe our experience in the diagnosis and treatment of AFBs retained Surgery, Mersin University, in the rectosigmoid colon. Materials and Methods: Between the years 2006 Abstract Mersin, 2Department of and 2015, a total of 11 patients diagnosed with AFBs were admitted to an General Surgery, Ondokuz emergency room and general surgery clinics. They were diagnosed and treated Mayıs University, Samsun, in four different hospitals in four different cities in Turkey. Information on the 3Department of General AFBs, clinical presentation, treatment strategies, and outcomes were documented. Surgery, Atatürk University, Erzurum, 4Department of We retrospectively reviewed the medical records of these unusual patients. General Surgery, Ordu Public Results: Eleven patients were involved in this study. All patients were male with Hospital, Ordu, 5Department their mean age was 49.81 (range, 23–71) years. The time of the presentation to of General Surgery, Faculty the removal of the foreign bodies ranged between 2 h and 96 h with a mean of of Medicine, Giresun 19.72 h. Ten patients inserted AFBs in the anus with the purpose of eroticism but University, Giresun, Turkey one patient’s reason to relieve constipation. -
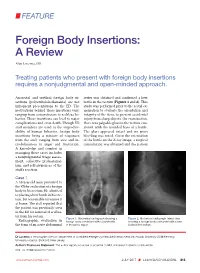
Foreign Body Insertions: a Review
FEATURE Foreign Body Insertions: A Review Alan Lucerna, DO Treating patients who present with foreign body insertions requires a nonjudgmental and open-minded approach. Anorectal and urethral foreign body in- series was obtained and confirmed a beer sertions (polyembolokoilamania) are not bottle in the rectum (Figures 1 and 2). This infrequent presentations to the ED. The study was performed prior to the rectal ex- motivations behind these insertions vary, amination to evaluate the orientation and ranging from autoeroticism to reckless be- integrity of the item, to prevent accidental havior. These insertions can lead to major injury from sharp objects. On examination, complications and even death. Though ED there was palpable glass in the rectum con- staff members are used to the unpredict- sistent with the rounded base of a bottle. ability of human behavior, foreign body The glass appeared intact and no gross insertions bring a mixture of responses bleeding was noted. Given the orientation from the staff, ranging from awe and in- of the bottle on the X-ray image, a surgical credulousness to anger and frustration. consultation was obtained and the patient A knowledge and comfort in managing these cases includes a nonjudgmental triage assess- ment, collective professional- ism, and self-awareness of the staff’s reaction. Case 1 A 58-year-old man presented to the ED for evaluation of a foreign body in his rectum. He admitted to placing a beer bottle in his rec- tum, but was unable to remove it at home. The staff reported that the patient was previously seen in the ED for removal of a vibra- tor from his rectum. -

Foreign Rectal Body – Systematic Review and Meta-Analysis
REVIEW 61 Foreign rectal body – Systematic review and meta-analysis M. Ploner1, A. Gardetto2, F. Ploner3, M. Scharl4, S. Shoap5, H. C. Bäcker5 (1) Department of Anesthesiology and Intensiver Care, Cantonal Spital Lucerne, Lucerne, Switzerland ; (2) Department of Plastic Surgery, Hospital Sterzing, Sterzing, South Tirol, Italy ; (3) Department of Anesthesiology and Emergency Medicine, Hospital Sterzing, South Tirol, Italy ; (4) Department of Gastroenterology and Hepatology, University Hospital Zurich, University of Zurich, Swetzerland ; (5) Department of Orthopaedic Surgery, Columbia University Medical Center, New York, USA. Abstract instrumentation (7). The most common complication is a rectal injury, which can result from a variety of agents and Background : Self-inserted foreign rectal bodies are an objects (8). Often, nonsurgical removal of foreign bodies infrequent occurrence, however they present a serious dilemma to the surgeon, due to the variety of objects, and the difficulty of has been described to be successful – in 11% to 65% – extraction. The purpose of this study is to give a comprehensive (9), however, in many situations, a surgical treatment review of the literature regarding the epidemiology, diagnostic may be essential. There have been a variety of algorithms tools and therapeutic approaches of foreign rectal body insertion. Methods : A comprehensive systematic literature review on introduced for the management of extraction, however, Pubmed/ Medline and Google for ‘foreign bodies’ was performed because of the diversity of foreign bodies, improvisation, on January 14th 2018. A meta-analysis was carried out to evaluate as well creativity of the treating emergency physician the epidemiology, diagnostics and therapeutic techniques. 1,551 abstracts were identified, of which 54 articles were included. -

Foreign Body Imaging-Experience with 6 Cases of Retained Foreign Bodies in the Emergency
Arch Clin Med Case Rep 2020; 4 (5): 952-968 DOI: 10.26502/acmcr.96550285 Case Series Foreign Body Imaging-Experience with 6 Cases of Retained Foreign Bodies in the Emergency Radiology Unit Muniraju Maralakunte MD1, Uma Debi MD1*, Lokesh Singh MD1, Himanshu Pruthi MD1, Vikas Bhatia MD, DNB DM1, Gita Devi MD1, Sandhu MS MD1 2Department of Radio diagnosis, PGIMER, Chandigarh, India *Corresponding Author: Dr. Uma Debi, Radiodiagnosis and Imaging, PGIMER, Chandigarh, India, Tel: 0091- 172-2756381; Fax: 0091-172-2745768; E-mail: [email protected] Received: 22 June 2020; Accepted: 14 August 2020; Published: 21 September 2020 Abstract Introduction: Retained foreign bodies are the external objects lying within the body, which are placed with voluntary or involuntary intentions. The involuntarily or accidentally, and complicated cases with the retained foreign body may come to the emergency services, which may require rapid and adequate imaging assessment. Materials and methods: We share our experience with six different cases with retained foreign bodies, who visited emergency radiological services with acute presentation of symptoms. The choice of radiological investigation considered based on the clinical presentation of the subjects with a retained foreign body. Conclusion: Patients with the retained foreign body may present acute symptoms to the emergency medical or surgical services, radiologists play a central role in rapid imaging evaluation. Radiological investigation plays a crucial role in identification, localization, characterization, and reporting the complication of the retained foreign bodies, and in many scenarios, radiological investigations may expose the unsuspected or concealed foreign bodies in the human body. Ultimately radiological services are useful rapid assessment tools that aid in triage and guide in the medical or surgical management of patients with a retained foreign body. -

Rectal Foreign Body: a Case Report
International Surgery Journal Cheereth RG et al. Int Surg J. 2017 Mar;4(3):1119-1122 http://www.ijsurgery.com pISSN 2349-3305 | eISSN 2349-2902 DOI: http://dx.doi.org/10.18203/2349-2902.isj20170874 Case Report Rectal foreign body: a case report Robin George Cheereth*, George Abraham Ninan Department of General Surgery, MorBaselios Medical Mission Hospital, Kothamangalam, Ernakulam 686691-Kerala, India Received: 27 December 2016 Accepted: 26 January 2017 *Correspondence: Dr. Robin George Cheereth, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Colorectal foreign bodies are infrequently encounteredand present a dilemma for management. The diagnosis may be confirmedby plain abdominal radiographs and rectal examination, butabdominal computerized tomography with 3-D reconstruction can be decisive in thefurther management and must be advised without reconsideration. Transanal removal is only possible for very low-lying objects, while patients with high-lying foreign bodiesusually require anoperative intervention. An early decision of laparotomy should only be madeafter subjecting the patient to suitable investigations to determineexactly the localization of the object, in order toavoid any inadvertent damage to the adjoining vasculatureas well as anal incontinence. We report the case of a youngadult male who presented in the emergency department with a Hand Held Bidet Shower inserted per rectum. Transanal removal was unsuccessful and Emergent laparotomy with colotomy and primary repair was necessary for safe removal of the same. -

Case Report Procedures Include Rectal Examination, Proctoscopy and Abdominal Radiography
Laparoscopic assisted removal of rectalrectal foreign bodybody Ashish Bhanot, G. R. Patel, Mitesh Bachani, Vijayraj D. Gohil Department of Surgery, Govt. Medical College, Bhavnagar, Gujarat, India For correspondence: Ashish Bhanot, C-7 Doctors Quarters, Sir T. Hospital Campus, Bhavnagar - 364 001, Gujarat, India. E-mail: [email protected] ABSTRACT ‘Foreign’ means originating elsewhere or simply ‘outside the body.’ Foreign body rectum is not as common as other parts of the body. Rectal foreign bodies present are difficult to manage. Emergency-department Case Report procedures include rectal examination, proctoscopy and abdominal radiography. Soft or low-lying objects having an edge could be grasped and removed safely in the emergency department, but grasping hard objects is potentially traumatic and occasionally results in upward migration toward the sigmoid. Although foreign bodies can be removed in the emergency department in about two out of three cases, some 10% still require a laparotomy and a diverting colostomy to remove the object or to treat bowel perforation. We are presenting a case of laparoscopic assisted removal of tumbler using 10 mm suction cannula to push the object down. Laparoscopy helped not only in retrieval but also enabled visualizing any bowel perforation due to foreign body and its manipulation. Key words: 10 mm suction cannula, foreign body rectum, laparoscopic assisted, sigmoid colostomy How to cite this article: Bhanot A, Patel GR, Bachani M, Gohil VD. Laparoscopic assisted removal of rectal foreign body. Indian J Surg 2006;68:216-8. INTRODUCTION blood were trickling from the anus. On finger examination, there was a reduced tone of anal sphincter Rectal foreign body, although infrequent, and circumference of glass tumbler could be reached by presents a challenge in management. -

The Medical Framing of Rectal Foreign Bodies
Culture, Health & Sexuality An International Journal for Research, Intervention and Care ISSN: 1369-1058 (Print) 1464-5351 (Online) Journal homepage: http://www.tandfonline.com/loi/tchs20 ‘Believe it or not’: the medical framing of rectal foreign bodies William J. Robertson To cite this article: William J. Robertson (2017): ‘Believe it or not’: the medical framing of rectal foreign bodies, Culture, Health & Sexuality To link to this article: http://dx.doi.org/10.1080/13691058.2016.1263874 Published online: 06 Jan 2017. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tchs20 Download by: [68.107.131.39] Date: 06 January 2017, At: 10:19 CULTURE, HEALTH, & SEXUALITY, 2017 http://dx.doi.org/10.1080/13691058.2016.1263874 ‘Believe it or not’: the medical framing of rectal foreign bodies William J. Robertson School of Anthropology, University of Arizona, Tucson, USA ABSTRACT ARTICLE HISTORY Medical and lay attention to and intervention for rectal foreign Received 14 June 2016 bodies, the presence of an object in the rectum most often via Accepted 19 November 2016 insertion through the anus, has long been a source of humour and KEYWORDS suspicion in both medical and public discourses. How do the ways Rectum; foreign bodies; medical providers write and talk to each other about rectal foreign disease framing; bodies shape and reflect understandings of gender, sexuality and the heteronormativity; medical (im)proper use of the anus and rectum? This paper examines the practice; medical literature medical literature on rectal foreign bodies to shed light on the ways in which medical providers frame rectal foreign bodies. -

Foreign Body Ingestion: Dos and Don'ts
ENDOSCOPY Frontline Gastroenterol: first published as 10.1136/flgastro-2020-101450 on 6 October 2020. Downloaded from EDUCATION IN PRACTICE Foreign body ingestion: dos and don’ts Aymeric Becq,1,2 Marine Camus,1,2 Xavier Dray 1,2 1Endoscopy Department, Hôpital INTRODUCTION Emesis, retching, blood- stained saliva, Saint Antoine, APHP, Sorbonne Foreign body ingestion comprises a true hypersialorrhoea, wheezing and/or respira- Université, Paris, Île- de- France, France foreign body (ie, non- food) ingestion and tory distress in non- communicative patients 2Paris On- call Endoscopy Team, food bolus impaction. Foreign body inges- (children and psychiatric patients) are Assistance Publique Hopitaux de tion is not uncommon and accounts for suggestive of foreign body impaction.7 Paris, Paris, Île- de- France, France roughly 4% of urgent endoscopies under- Oesophageal impaction (food bolus) is often 1 2 Correspondence to taken. True foreign body ingestion is symptomatic: retching, vomiting, foreign Professor Xavier Dray, Endoscopy mostly encountered in paediatric popula- body sensation, dysphagia, odynophagia, Unit, Hôpital Saint Antoine, APHP, tions with 75% of cases occurring in less sore throat and retrosternal pain. Hypersia- Sorbonne Université, 75012 1 Paris, Île- de- France, France; than 5- year- old children. Coins, buttons, lorrhoea and inability to manage secretions xavier. dray@ aphp. fr plastic items, batteries and bones are suggest complete oesophageal obstruction, common culprits.3 Food bolus impaction warranting urgent endoscopic retrieval.7 Received 1 July 2020 Revised 27 August 2020 on the other hand is mostly seen in adults, Cervical crepitus, neck swelling or pneumo- Accepted 30 August 2020 usually accidental (95% of cases). Steakhouse mediastinum are suggestive of oesophageal syndrome, animal bones, toothpicks and fish perforation. -

Case Report a Rare Case of Presence of Unusual
Case Report A rare case of presence of unusual foreign body in rectum in homosexual male Vaibhav Vishal1, Naresh Meena2, Manish Mahendra3 From 1Senior Resident, Department of Urology, Calicut Medical College, Kozhikode, Kerala, 2Assistant Professor, Department of General surgery, Bikaner Medical College, Bikaner, Rajasthan, 3Senior Resident, Department of Neuro surgery, Dr. Baba Sahib Ambedkar Hospital, New Delhi, India Correspondence to: Vaibhav Vishal, S/O Dr. D K Shrivastava, Global Hospital, Mount Abu - 307 501, Rajasthan, India. E-mail: [email protected] Received – 09 April 2018 Initial Review – 29 April 2018 Published Online – 21 June 2018 ABSTRACT Foreign body in the rectum is rare yet potentially life-threatening condition; fear of social embarrassment causes the patient to delay to seek treatment and even hide the actual history and replace imaging reports, which leads to incorrect diagnosis and eventually the treatment. In this article, we report the case of a 30 years hepatitis B positive, homosexual male who presented to the department with a chief complaint of pain in the lower abdomen and was diagnosed to have foreign body (pestle) in rectum, which was inserted as a part of sexual adventure and deliberate attempt was made by the patient to replace X-ray films with other patients to mislead the treating doctor. Finally, the foreign body was removed surgically and was followed by psychiatric evaluation of the patient. Key words: Foreign body, Rectum, Sexual misadventure, X-ray films he presence of foreign body in the rectum is rare yet On clinical examination, vital signs were within the normal potentially life-threating condition and its incidence has range. -

Management of Rectal Foreign Bodies Koornstra, Jan J.; Weersma, Rinse K
University of Groningen Management of rectal foreign bodies Koornstra, Jan J.; Weersma, Rinse K. Published in: World Journal of Gastroenterology DOI: 10.3748/wjg.14.4403 IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2008 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Koornstra, J. J., & Weersma, R. K. (2008). Management of rectal foreign bodies: Description of a new technique and clinical practice guidelines. World Journal of Gastroenterology, 14(27), 4403-4406. https://doi.org/10.3748/wjg.14.4403 Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). The publication may also be distributed here under the terms of Article 25fa of the Dutch Copyright Act, indicated by the “Taverne” license. More information can be found on the University of Groningen website: https://www.rug.nl/library/open-access/self-archiving-pure/taverne- amendment. Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum.