Populations in Surrey, British Columbia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

City of Surrey 2012 - 2021 Dog Off Leash Area Strategy
TABLE OF CONTENTS 1.0 INTRODUCTION 3 4.0 OPERATE 93 1.1 EXECUTIVE SUMMARY 4 4.1 MAINTENANCE 94 1.2 SUSTAINABILITY CHARTER 21 4.2 WASTE MANAGEMENT 95 4.3 COMMUNITY ENGAGEMENT 100 2.0 PLAN 23 4.4 PRIVATELY-RUN DOG PARKS 102 2.1 RATIONALE FOR OFF LEASH AREAS 25 4.5 OFF LEASH AREA CODE OF CONDUCT 103 2.2 learning from surrey’s 4.6 ENFORCEMENT + SELF-POLICING 104 EXISTING OFF LEASH AREAS 26 4.6 MONITORING + ASSESSMENT 106 2.3 QUALITIES OF SUCCESSFUL DOG PARKS 30 4.7 BEST MANAGEMENT PRACTICES FOR 2.4 HEALTH, SAFETY AND THE SURREY OFF LEASH AREAS 108 ENVIRONMENT 32 4.8 SUGGESTED PILOT PROJECTS: 2.5 USE OF HYDRO RIGHT OF WAYS 37 OFF-SITE COMPOSTING OF DOG WASTE + ANAEROBIC DIGESTION 110 2.6 LOCATION AND PROVISION GUIDELINE PRECEDENTS 39 2.7 PROVISION + LOCATION GUIDELINES 40 5.0 RESOURCES 113 2.8 RECOMMENDED LOCATIONS FOR SURREY 5.1 REFERENCES 114 OFF LEASH AREAS 42 5.2 MUNICIPALITIES WITH DOG PARK PLANS 116 3.0 DESIGN 49 6.0 APPENDICES 119 3.1 OFF LEASH AREA AMENITIES 50 APPENDIX 1.0: STAFF WORKSHOP 121 3.2 SPACE ALLOCATION 52 APPENDIX 2.0: STAKEHOLDER WORKSHOP 141 3.3 SURFACE MATERIALS 54 APPENDIX 3.0: PHONE SURVEY 155 3.4 EDGE CONDITIONS 58 APPENDIX 4.0: ONLINE SURVEY 179 3.5 DESIGN GUIDELINES FOR CITY OF SURREY OFF LEASH AREAS 60 APPENDIX 5.0: OPEN HOUSE SERIES 1 185 3.6 OFF LEASH AREA DESIGN CONCEPTS 64 APPENDIX 6.0: OPEN HOUSE SERIES 2 211 3.7 SUGGESTED PILOT PROJECT: REPURPOSING ARTIFICIAL TURF 91 Cover page photo source: flickr user nruebotham CITY OF SURREY 2012 - 2021 DOG OFF LEASH AREA STRATEGY ABOUT ONE-THIRD OF ALL SURREY DOG OWNERS VISIT A DESIGNATED OFF LEASH AREA IN SURREY EACH WEEK. -

9930 197Th Street, Langley, Bc Port Kells Industrial Area
FOR SALE 14.54 ACRE INDUSTRIAL DEVELOPMENT SITE 9930 197TH STREET, LANGLEY, BC PORT KELLS INDUSTRIAL AREA GOLDEN EARS BRIDGE GOLDEN EARS WAY CHRIS MACCAULEY JOE INKSTER PERSONAL REAL ESTATE CORPORATION PERSONAL REAL ESTATE CORPORATION 604.662.5190 604.662.5134 [email protected] [email protected] OPPORTUNITY CBRE has been engaged by Trimac Transportation to facilitate this exceedingly rare opportunity to list for sale industrial land in Metro Vancouver. THE LOCATION The subject property is located in the well established Port Kells industrial area, and is considered the Industrial Hub of the lower mainland. Port Kells (781.1 acres) is located on the border of Surrey and Langley, on the south side of the Fraser River, north of Highway 1 and east of Highway 15. The industrial lands are in high demand due to the area’s strategic location within Metro Vancouver and access to key transportation corridors including the east/west Highway 1 and the north/south Highway 15 that provides a direct link to the US commercial border crossing. The South Fraser CIVIL & LEGAL ADDRESSES Perimeter Road (SFPR) provides excellent access to the port, the gateway to Asia Pacific. Port Kells is home to a diverse range of industries with a high 9930 197TH STREET, LANGLEY, BC concentration of manufacturing, PID: 001-618-148 PARCEL A (Y68082) DISTRICT LOT 122 GROUP 2 NEW WESTMINSTERDISTRICT PLAN 54498 TENANCY Lafarge Canada currently leases 4.013 acres from Trimac Transportation. SITE SIZE Trimac Transportation will also consider a leaseback of the property. 14.54 ACRES PROPERTY TAXES (2018) SUBMISSION GUIDELINE $349,755.40 Potential purchasers that require access to additional information such as environmental documents, site plans, purchase and sale agreement, leases, and leaseback agreement, must complete the non-disclosure agreement. -

SURREY RELOCATION GUIDE: Doing Business in Surrey
SURREY BOARD OF TRADE FURTHERING THE INTERESTS OF BUSINESSES SINCE 1918 Surrey Board of Trade SURREY RELOCATION GUIDE: Doing Business in Surrey Surrey Board of Trade supports and attracts companies by: ■ Providing data on all aspects of local ■ Connecting universities and colleges that infrastructure including highway and air offer customized training programs for new connections, human capital and commercial workers to match industry needs to curriculum real estate. development. ■ Identifying real estate options including ■ Providing information and facilitating contacts office space for new building construction in with federal, provincial & local authorities. collaboration with local real estate firms. Exploring financial incentive programs and ■ Business match-making. venture capital programs. ■ Developing contacts with foreign-owned ■ Conducting city tours. companies. businessinsurrey.com SURREY BOARD OF TRADE Overview Surrey’s business landscape, growth opportunities and why it’s a prime location for businesses urrey is known for being one thing: WHERE SURREY STANDS WITHIN METRO VANCOUVER a powerful, Population by each municipality in Metro Vancouver (2011 v. 2015) progressive economic engine of Metro Vancouver in which to do business and invest. SIt could be the largest city in British Columbia in the next 20 years. Surrey’s population, which currently sits at almost 520,000, is projected to increase by an additional 250,000 people in the next 30 years. By 2041, one in five Metro Vancouver residents will live in Surrey. It has the highest median family income, it is centrally located between the commercial hub of Vancouver and the U.S. border (in fact, Surrey is a U.S. border city) and it is close to two international airports. -

Discovery Guide Are Filled with Valuable Map (Centrefold) 12 Information to Help You with Your Stay
Discovery2018-2019 Guide DISCOVERSURREYBC.COM discoversurreybc.com Front cover photo: Damon West @damonwestphotography www.damonwestphotography.com Welcome to Surrey! There are many reasons to visit our growing city. Whether you’re here for an afternoon or a two-week vacation, we want to make sure your Surrey story is filled with adventure, delicious food, and unforgettable experiences. Inside Regardless of what brings you to Surrey, one thing’s for certain, there are so many things to discover here. As a city, Surrey may be vast in size, but when it comes down to the nitty-gritty, we like Festivals and Events 4 to think we’ve got a cozy, small-town feel. Maybe it’s our sense of South Surrey 6 community, our civic pride, or the fact that Surrey is made up of six unique neighbourhoods. Discover Surrey by its neighbourhoods North Surrey 8 and find out which one suits you best! Cloverdale 10 The pages of this Discovery Guide are filled with valuable Map (Centrefold) 12 information to help you with your stay. If you need further Fleetwood 14 assistance with your travel planning requirements, our website www.discoversurreybc.com is a great source of tips and Newton 16 information. Guildford 18 Parks 20 Share your Culinary 21 #TrueSurrey Golf 22 Accommodation 23 Find us online @discoversurreybc [email protected] The Official Discovery Guide is produced by Discover Surrey to promote tourism in the community. No part of this publi- cation may be reproduced without written permission from Discover Surrey. Publishers accept no responsibility for errors or omissions. -

Vancouver Canada Public Transportation
Harbour N Lions Bay V B Eagle I P L E 2 A L A 5 A R C Scale 0 0 K G H P Legend Academy of E HandyDART Bus, SeaBus, SkyTrain Lost Property Customer Service Coast Express West Customer Information 604-488-8906 604-953-3333 o Vancouver TO HORSESHOE BAY E n Local Bus Routes Downtown Vancouver 123 123 123 i CHESTNUT g English Bay n l Stanley Park Music i AND LIONS BAY s t H & Vancouver Museum & Vancouver h L Anthropology Beach IONS B A A W BURRARD L Y AV BURRARD Park Museum of E B t A W Y 500 H 9.16.17. W 9 k 9 P Y a Lighthouse H.R.MacMillan G i 1 AVE E Vanier n Space Centre y r 3 AVE F N 1 44 Park O e s a B D o C E Park Link Transportation Major Road Network Limited Service Expo Line SkyTrain Exchange Transit Central Valley Greenway Central Valley Travel InfoCentre Travel Regular Route c Hospital Point of Interest Bike Locker Park & Ride Lot Peak Hour Route B-Line Route & Stop Bus/HOV Lane Bus Route Coast Express (WCE) West Millennium Line SkyTrain Shared Station SeaBus Route 4.7.84 A O E n Park 4 AVE 4 AVE l k C R N s H Observatory A E V E N O T 2 e S B University R L Caulfeild Columbia ta Of British Southam E 5 L e C C n CAULFEILD Gordon Memorial D 25 Park Morton L Gardens 9 T l a PINE 253.C12 . -

Hawthorne Park.Pdf
From: Sarah F. Alger To: Andrew Vorce Cc: Vicki S. Marsh ([email protected]); Megan Trudel; Holly Visco; Terry Sanford ([email protected]); Judith Wegner Subject: Hawthorne Park Date: Friday, March 6, 2020 12:22:29 AM Dear Andrew, I was surprised to learn that the Hawthorne Park matter was discussed by the Board at its meeting today. Based upon my conversation with staff and a review of the agenda, I understood that this matter was continued until Monday night. My experience has been that when an item on the agenda is specifically marked as being continued to a date certain, as was the case here, that is notice to the public and applicants alike that the matter is off the agenda for that meeting and will not be discussed. Had we known that the matter would be discussed at today’s meeting, both my client and I certainly would have attended. That said, I understand that the Board has raised the following questions that it hopes can be addressed at or before the meeting Monday evening. We are happy to answer any questions that the Board or any interested party might have. The questions posed by the Board are below in blue, and my responses are in red. 1. What are the consequences if the Board declines to approve signing this document? I do not believe that there would be any consequences if the Board declined to approve the proposed document. The language of the special permit is clear that the permit is not affected by the failure of the applicant to find a holder for the Conservation Restriction (“CR”) as long as the Board finds that the applicant has made a good faith effort. -

Historical Heritage Study
PATTULLO BRIDGE REPLACEMENT PROJECT EAC APPLICATION Note to the Reader This report was finalized before the Pattullo Bridge Replacement Project was transferred from TransLink (South Coast British Columbia Transportation Authority) to the BC Ministry of Transportation and Infrastructure (MoTI). References to TransLink should be read as MoTI unless referring specifically to TransLink policies or other TransLink-related aspects. Translink Hatfield Consultants Pattullo Bridge Replacement Project Historical Heritage Study April 2018 Submitted by: Denise Cook Design Team: Denise Cook, Denise Cook Design Project contact: Denise Cook, CAHP Principal, Denise Cook Design #1601-1555 Eastern Avenue North Vancouver BC V7N 2X7 Telephone: 604-626-2710 Email: [email protected] TABLE OF CONTENTS 1.0 INTRODUCTION .............................................................................. 1 2.0 OBJECTIVES .................................................................................... 1 3.0 METHODOLOGY ............................................................................. 3 3.1 General Methodology ....................................................... 3 3.2 Planning and policy context .............................................. 4 4.0 HISTORICAL CONTEXT .................................................................... 4 4.1 Brief Historical Context of Bridge and Environs ............... 4 4.2 Early Land Uses ................................................................. 5 4.3 Transportation networks ............................................... -

Handbook of Biophilic City Planning and Design
Handbook of Biophilic City Planning & Design TIMOTHY BEATLEY Handbook of Biophilic City Planning and Design Handbook of Biophilic City Planning and Design Timothy Beatley Washington | Covelo | London Copyright © 2016 Timothy Beatley All rights reserved under International and Pan-American Copyright Conventions. No part of this book may be reproduced in any form or by any means without permission in writing from the publisher: Island Press, 2000 M Street, NW, Suite 650, Washington, DC 20036 ISLAND PRESS is a trademark of the Center for Resource Economics. Library of Congress Control Number: 2016938091 Printed on recycled, acid-free paper Manufactured in the United States of America 10 9 8 7 6 5 4 3 2 1 Keywords: Biodiversity, Biophilia, Biophilic Cities Network, bird-safe buildings, Birmingham (United Kingdom), carbon footprint, climate change, ecosystem, garden, greenbelt, green infrastructure, Green Streets Initiative, health, Intertwine, Khoo Teck Puat Hospital, Milwaukee, nature, nature center, Oslo, park, parklet, Portland, resilience, restoration, San Francisco, Singapore, Stephen Kellert, Sutton Park, urban ecology, vertical garden, Vitoria- Gastiez (Spain), watershed, Wellington (New Zealand), Zealandia To Anneke, Caro, and Jadie Contents List of Case Studies .............................................................................................................. xi Preface .................................................................................................................................xv Acknowledgments ................................................................................................................xix -
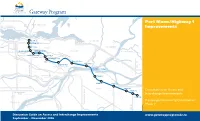
Port Mann/Highway 1 Improvements
Port Mann/Highway 1 Improvements McGill St. Hastings St. First Ave. Port Coquitlam Boundary Rd. Willingdon Ave. Grandview Hwy. Sprott St. Kensington Ave. Brunette Ave. Cape Horn Gaglardi Way Port Mann Bridge 152nd St. 160th St. 176th St. 192nd St. 200th St. Consultation on Access and Interchange Improvements 216th St. Pre-design Community Consultation Phase 2 200 St Discussion Guide on Access and Interchange Improvements www.gatewayprogram.bc.ca September – November 2006 CONSULTATION ON ACCESS AND INTERCHANGE IMPROVEMENTS: PRE-DESIGN COMMUNITY CONSULTATION PHASE 2 The Ministry of Transportation conducts community consultations at three design stages, including: Pre-design Consultation: Phase 1 Pre-design Consultation: Phase 2 Preliminary Design Consultation Detailed Design Consultation February – April 2006 September – November 2006 2008 2009 Pre-design consultation discussion topics This phase of consultation focuses on With basic pre-design components Detailed design consultation generally focuses included goals for interchange upgrades, proposed conceptual improvements determined, consultation on preliminary on fewer but more specifi c treatments, such congestion reduction measures such as high to existing and new interchanges and design discusses refi nements to interchanges as detailed interchange and access features, occupancy vehicle lanes (HOV) and transit overpasses. These proposed modifi cations and accesses, lane use, specifi cs of congestion lighting and landscaping. This phase also priority on-ramps, commercial vehicle priority and upgrades would improve safety, access reduction measures and other key features. involves more fi nancial and technical analysis access to on-ramps, potential tolling, and and connections across the highway, support to confi rm that designs are fi nancially and improvements to the cycling network. -

Grand Junction Parks & Recreation
Grand Junction Parks & Recreation Parks Trails Trees Cemeteries Golf Courses Art Aquatics Shelters The Grand Junction Parks and Recreation Department is excited to present this updated facility guide to all of our local residents and visitors. You will find easy access to our parks, trails, golf courses and more. The mission statement for the City of Grand Junction is “to be the most livable community west of the Rockies by 2025.” The programs and facilities provided by the Parks and Recreation Department will play an integral role in the success of achieving that mission. The energetic staff is dedicated to providing a wide variety of parks and facilities that appeal to all ages while also offering a broad range of recreational amenities. The Department has been recognized as a Gold Medal winner by the National Recreation and Parks Association for our outstanding service to the public. Throughout this guide, you will find access to Grand Junction’s developed and undeveloped parks, community aquatic centers, municipal cemeteries, developed trail systems, and golf courses. For your convenience, we have developed a user-friendly chart in the center of the guide highlighting all of the major park amenities throughout our system. If you are looking specifically for a dog park, skateboard park, or tennis court, this chart will provide quick access at a glance. Also included in this booklet, you will find the “Urban Forestry Guide” and “Art in the Parks.” The forestry guide will provide you with good planting and care practices and highlights many of the more commonly grown species throughout the valley. -

La Ville Insaisissable the Elusive City
SPRING | PRINTEMPS 2013 vol.15_ no. 2 | 8.00$ la villethe insaisissable elusive city THE CANADIAN SOCIETY OF LANDSCAPE ARCHITECTS SOCIETY OF LANDSCAPE THE CANADIAN DU CANADA PAYSAGISTES DES ARCHITECTES L’ASSOCIATION www.csla-aapc.ca DESIGN for the COMMUNITY DESIGN pour la COLLECTIVITÉ TaTablbleses PT-T 2,2 Pououbbeelllleses S-4-42.2. Insnsttaalllattion à Londdreres, en AnAnglg etterre. We design, engineer and manufacture long-lasting, ergonomic and attractive site furnishings. For your convenience, our quotes are in Canadian dollars and our shipments move by truck directly to your delivery site. Victor Stanley, Inc. conçoit, met en œuvre et fabrique des mobiliers extérieurs durables, ergonomiques et attrayants. Pour accommoder nos clients du Canada, nos devis sont faits en dollars canadiens, et nos produits sont livrés par camion là où vous le désirez. 1.800.368.2573 (Canada & USA) | Maryland, USA | www.victorstanley.com 627166_Victor.indd 1 01/02/13 2:58 AM 621519_Leader.indd 1 22/12/12 12:20 AM 629981_Vitamin.indd 1 02/03/13 6:08 PM Learn more at playlsi.com/ad/custom-la or call 888.438.6574 or 763.972.5200. ©2012 Landscape Structures Inc. 622097_Landscape.indd 1 06/01/13 1:40 AM 622679_Manderley.indd 1 11/01/13 3:47 AM CANADA’S FIRST QUALIFIED 100% CERTIFIED DROUGHT-TOLERANT SOD SEED BLEND Request your FREE copy of the LEED Guidance Letter for Drought-Tolerant Grass by Morrison Hershfield. [email protected] or 613.225.7500 x229 622097_Landscape.indd 1 06/01/13 1:40 AM 622679_Manderley.indd 1 11/01/13 3:47 AM Hawthorne Park | Philadelphia, -
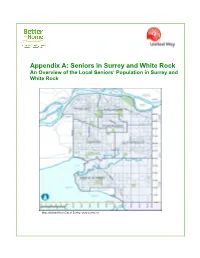
Appendix A: Seniors in Surrey and White Rock an Overview of the Local Seniors’ Population in Surrey and White Rock
Appendix A: Seniors in Surrey and White Rock An Overview of the Local Seniors’ Population in Surrey and White Rock Map obtained from City of Surrey: www.surrey.ca Better at Home: Seniors in Surrey and White Rock Introduction The purpose of this Brief is to present an overview of the local seniors’ population in Surrey and White Rock, with a view to helping the United Way of the Lower Mainland (“UWLM”), through it’s Better at Home1 program, understand where seniors live, how many are isolated and/or vulnerable and how many require additional help at home. As a first step in the community development process associated with the Better at Home program in Surrey/White Rock, this Brief provides a snapshot of the seniors’ population based on secondary research. The research presented here is supplemented by additional local research including public surveys and interviews2. This Paper contains: a) An overview of the seniors’ population in Metro Vancouver; b) An overview of the seniors’ population in Surrey and White Rock in general; and, c) A snapshot of Surrey’s and White Rock’s senior populations by neighbourhood/community, including select demographic information as to where they live, income, ethnicity and general vulnerability. 1. The Regional Context: Seniors in Metro Vancouver Metro Vancouver has had substantial population growth since the 2006 Census year. The population increased by 197,000 people for a total population in 2011 of 2,313,328. Surrey is the second largest municipality in Metro Vancouver with a 2011 population of 468,251 (20% of the region’s population).