Checklist of the Myriapoda in Cretaceous Burmese Amber and a Correction of the Myriapoda Identified by Zhang (2017)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diplopoda: Polydesmida)
Records of the Hawaii Biological Survey for 1997—Part 2: Notes 43 P-0244 HAWAI‘I: East slope of Mauna Loa, Kïpuka Ki Weather Station, 1220 m, pitfall trap, 10–12.iv.1972, J. Jacobi P-0257 HAWAI‘I: East slope of Mauna Loa, 1280–1341 m, pitfall trap, 8–10.v.1972, J. Jacobi P-0268 HAWAI‘I: East slope of Mauna Loa, 1890 m, pitfall trap, 5–7.vi.1972, J. Jacobi P-0269 HAWAI‘I: East slope of Mauna Loa, 1585 m, pitfall trap, 5–7.vi.1972, J. Jacobi P-0271 HAWAI‘I: East slope of Mauna Loa, 1280–1341 m, pitfall trap, 5–7.vi.1972, J. Jacobi P-0281 HAWAI‘I: East slope of Mauna Loa, 1981 m, pitfall trap, 10–12.vii.1972, J. Jacobi P-0284 HAWAI‘I: East slope of Mauna Loa, 1585 m, pitfall trap, 10–12.vii.1972, J. Jacobi P-0286 HAWAI‘I: East slope of Mauna Loa, Kïpuka Ki Weather Station, 1220 m, pitfall trap, 10–12.vii.1971, J. Jacobi P-0291 HAWAI‘I: East slope of Mauna Loa, Kilauea Forest Reserve, 1646 m, pitfall trap, 10–12.vii.1972 J. Jacobi P-0294 HAWAI‘I: East slope of Mauna Loa, 1981 m, pitfall trap, 14–16.viii.1972, J. Jacobi P-0300 HAWAI‘I: East slope of Mauna Loa, Kilauea Forest Reserve, 1646 m, pitfall trap, J. Jacobi P-0307 HAWAI‘I: East slope of Mauna Loa, 1981 m, pitfall trap, 17–19.ix.1972, J. Jacobi P-0313 HAWAI‘I: East slope of Mauna Loa, Kilauea Forest Reserve, 1646 m, pitfall trap, 17–19.x.1972, J. -

Millipedes (Diplopoda) from Caves of Portugal
A.S.P.S. Reboleira and H. Enghoff – Millipedes (Diplopoda) from caves of Portugal. Journal of Cave and Karst Studies, v. 76, no. 1, p. 20–25. DOI: 10.4311/2013LSC0113 MILLIPEDES (DIPLOPODA) FROM CAVES OF PORTUGAL ANA SOFIA P.S. REBOLEIRA1 AND HENRIK ENGHOFF2 Abstract: Millipedes play an important role in the decomposition of organic matter in the subterranean environment. Despite the existence of several cave-adapted species of millipedes in adjacent geographic areas, their study has been largely ignored in Portugal. Over the last decade, intense fieldwork in caves of the mainland and the island of Madeira has provided new data about the distribution and diversity of millipedes. A review of millipedes from caves of Portugal is presented, listing fourteen species belonging to eight families, among which six species are considered troglobionts. The distribution of millipedes in caves of Portugal is discussed and compared with the troglobiont biodiversity in the overall Iberian Peninsula and the Macaronesian archipelagos. INTRODUCTION All specimens from mainland Portugal were collected by A.S.P.S. Reboleira, while collectors of Madeiran speci- Millipedes play an important role in the decomposition mens are identified in the text. Material is deposited in the of organic matter, and several species around the world following collections: Zoological Museum of University of have adapted to subterranean life, being found from cave Copenhagen, Department of Animal Biology, University of entrances to almost 2000 meters depth (Culver and Shear, La Laguna, Spain and in the collection of Sofia Reboleira, 2012; Golovatch and Kime, 2009; Sendra and Reboleira, Portugal. 2012). Although the millipede faunas of many European Species were classified according to their degree of countries are relatively well studied, this is not true of dependence on the subterranean environment, following Portugal. -
Integrative Revision of the Giant Pill-Millipede Genus Sphaeromimus
A peer-reviewed open-access journal ZooKeys 414: 67–107 (2014) New Sphaeromimus species from Madagascar 67 doi: 10.3897/zookeys.414.7730 RESEARCH ARTICLE www.zookeys.org Launched to accelerate biodiversity research Integrative revision of the giant pill-millipede genus Sphaeromimus from Madagascar, with the description of seven new species (Diplopoda, Sphaerotheriida, Arthrosphaeridae) Thomas Wesener1,2,†, Daniel Minh-Tu Le1,3,‡, Stephanie F. Loria1,4,§ 1 Field Museum of Natural History, Zoology - Insects, 1400 S. Lake Shore Drive, 60605 Chicago, Illinois, U.S.A. 2 Zoologisches Forschungsmuseum Alexander Koenig, Leibniz Institute for Animal Biodiversity, Center for Taxonomy and Evolutionary Research (Section Myriapoda), Adenauerallee 160, 53113 Bonn, Germany 3 School of the Art Institute of Chicago, 36 S. Wabash Avenue, 60603 Chicago, Illinois, U.S.A. 4 American Museum of Natural History, Richard Glider Graduate School, Central Park West at 79th Street, New York, U.S.A. † http://zoobank.org/86DEA7CD-988C-43EC-B9D6-C51000595B47 ‡ http://zoobank.org/AD76167C-3755-4803-AEB5-4CD9A7CB820A § http://zoobank.org/ED92B15A-10F9-47B8-A8FA-D7673007F8A5 Corresponding author: Thomas Wesener ([email protected]) Academic editor: D.V. Spiegel | Received 15 April 2014 | Accepted 8 May 2014 | Published 6 June 2014 http://zoobank.org/59FA2886-34C2-4AEF-9783-3347E5EBC702 Citation: Wesener T, Le DM-T, Loria SF (2014) Integrative revision of the giant pill-millipede genus Sphaeromimus from Madagascar, with the description of seven new species (Diplopoda, Sphaerotheriida, Arthrosphaeridae). ZooKeys 414: 67–107. doi: 10.3897/zookeys.414.7730 Abstract The Malagasy giant pill-millipede genusSphaeromimus de Saussure & Zehntner, 1902 is revised. Seven new species, S. -

Supra-Familial Taxon Names of the Diplopoda Table 4A
Milli-PEET, Taxonomy Table 4 Page - 1 - Table 4: Supra-familial taxon names of the Diplopoda Table 4a: List of current supra-familial taxon names in alphabetical order, with their old invalid counterpart and included orders. [Brackets] indicate that the taxon group circumscribed by the old taxon group name is not recognized in Shelley's 2003 classification. Current Name Old Taxon Name Order Brannerioidea in part Trachyzona Verhoeff, 1913 Chordeumatida Callipodida Lysiopetalida Chamberlin, 1943 Callipodida [Cambaloidea+Spirobolida+ Chorizognatha Verhoeff, 1910 Cambaloidea+Spirobolida+ Spirostreptida] Spirostreptida Chelodesmidea Leptodesmidi Brölemann, 1916 Polydesmida Chelodesmidea Sphaeriodesmidea Jeekel, 1971 Polydesmida Chordeumatida Ascospermophora Verhoeff, 1900 Chordeumatida Chordeumatida Craspedosomatida Jeekel, 1971 Chordeumatida Chordeumatidea Craspedsomatoidea Cook, 1895 Chordeumatida Chordeumatoidea Megasacophora Verhoeff, 1929 Chordeumatida Craspedosomatoidea Cheiritophora Verhoeff, 1929 Chordeumatida Diplomaragnoidea Ancestreumatoidea Golovatch, 1977 Chordeumatida Glomerida Plesiocerata Verhoeff, 1910 Glomerida Hasseoidea Orobainosomidi Brolemann, 1935 Chordeumatida Hasseoidea Protopoda Verhoeff, 1929 Chordeumatida Helminthomorpha Proterandria Verhoeff, 1894 all helminthomorph orders Heterochordeumatoidea Oedomopoda Verhoeff, 1929 Chordeumatida Julida Symphyognatha Verhoeff, 1910 Julida Julida Zygocheta Cook, 1895 Julida [Julida+Spirostreptida] Diplocheta Cook, 1895 Julida+Spirostreptida [Julida in part[ Arthrophora Verhoeff, -

Local and Regional Influences on Arthropod Community
LOCAL AND REGIONAL INFLUENCES ON ARTHROPOD COMMUNITY STRUCTURE AND SPECIES COMPOSITION ON METROSIDEROS POLYMORPHA IN THE HAWAIIAN ISLANDS A DISSERTATION SUBMITTED TO THE GRADUATE DIVISION OF THE UNIVERSITY OF HAWAI'I IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN ZOOLOGY (ECOLOGY, EVOLUTION AND CONSERVATION BIOLOGy) AUGUST 2004 By Daniel S. Gruner Dissertation Committee: Andrew D. Taylor, Chairperson John J. Ewel David Foote Leonard H. Freed Robert A. Kinzie Daniel Blaine © Copyright 2004 by Daniel Stephen Gruner All Rights Reserved. 111 DEDICATION This dissertation is dedicated to all the Hawaiian arthropods who gave their lives for the advancement ofscience and conservation. IV ACKNOWLEDGEMENTS Fellowship support was provided through the Science to Achieve Results program of the U.S. Environmental Protection Agency, and training grants from the John D. and Catherine T. MacArthur Foundation and the National Science Foundation (DGE-9355055 & DUE-9979656) to the Ecology, Evolution and Conservation Biology (EECB) Program of the University of Hawai'i at Manoa. I was also supported by research assistantships through the U.S. Department of Agriculture (A.D. Taylor) and the Water Resources Research Center (RA. Kay). I am grateful for scholarships from the Watson T. Yoshimoto Foundation and the ARCS Foundation, and research grants from the EECB Program, Sigma Xi, the Hawai'i Audubon Society, the David and Lucille Packard Foundation (through the Secretariat for Conservation Biology), and the NSF Doctoral Dissertation Improvement Grant program (DEB-0073055). The Environmental Leadership Program provided important training, funds, and community, and I am fortunate to be involved with this network. -

A New Genus of the Millipede Tribe Brachyiulini (Diplopoda: Julida: Julidae) from the Aegean Region
European Journal of Taxonomy 70: 1-12 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2013.70 www.europeanjournaloftaxonomy.eu 2013 · Lazányi E. & Vagalinski B. This work is licensed under a Creative Commons Attribution 3.0 License. Research article urn:lsid:zoobank.org:pub:5E23F454-2A68-42F6-86FB-7D9952B2FE7B A new genus of the millipede tribe Brachyiulini (Diplopoda: Julida: Julidae) from the Aegean region Eszter LAZÁNYI1,4 & Boyan VAGALINSKI2,3,5 1 Corresponding author: Department of Zoology, Hungarian Natural History Museum, Baross u. 13, H-1088 Budapest, Hungary. E-mail: [email protected] 2 Faculty of Biology, Sofia University, 8 Dragan Tsankov Blvd., 1164 Sofia, Bulgaria. E-mail: [email protected] 3 Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, 2 Yurii Gagarin Street, 1113, Sofia, Bulgaria. 4 urn:lsid:zoobank.org:author:02DB48F1-624C-4435-AF85-FA87168CD85A 5 urn:lsid:zoobank.org:author:973B8725-039E-4F29-8D73-96A7F52CF934 Abstract. A new genus of the julid tribe Brachyiulini, Enghophyllum gen. nov., is described, comprising two species from Greece. The type-species, E. naxium (Verhoeff, 1901) comb. nov. (ex Megaphyllum Verhoeff, 1894), appears to be rather widespread in the Aegean: it is known from Antiparos Island and Naxos Island (the type locality), both in the Cyclades, as well as East Mavri Islet, Dodecanese Archipelago (new record). The vulva of E. naxium is described for the first time. In addition,E. sifnium gen. et sp. nov. is described based on a single adult male from Sifnos Island, Cyclades. The new genus is distinct from other genera of the Brachyiulini mainly by its peculiar gonopod structure, apparently disjunct and at least mostly apomorphous: (1) promeres broad, shield-like, in situ protruding mostly posteriad, completely covering the opisthomeres and gonopodal sinus; (2) transverse muscles and coxal apodemes of promere fully reduced; (3) opisthomere with three differentiated processes, i.e., lateral, basal posterior and apical posterior; (4) solenomere rather simple, tubular. -

Type and Figured Fossils in the Worthen Collection at the Illinois
s Cq&JI ^XXKUJtJLI 14oGS: CIR 524 c, 2 TYPE AND FIGURED FOSSILS IN THE WORTHEN COLLECTION AT THE ILLINOIS STATE GEOLOGICAL SURVEY Lois S. Kent GEOLOGICAL ILLINOIS Illinois Department of Energy and Natural Resources, STATE GEOLOGICAL SURVEY DIVISION CIRCULAR 524 1982 COVER: This portrait of Amos Henry Worthen is from a print presented to me by Worthen's great-grandson, Arthur C. Brookley, Jr., at the time he visited the Illinois State Geological Survey in the late 1950s or early 1960s. The picture is the same as that published in connection with the memorial to Worthen in the appendix to Vol. 8 of the Geological Survey of Illinois, 1890. -LSK Kent, Lois S., Type and figured fossils in the Worthen Collection at the Illinois State Geological Survey. — Champaign, III. : Illinois State Geological Survey, 1982. - 65 p. ; 28 cm. (Circular / Illinois State Geological Survey ; 524) 1. Paleontology. 2. Catalogs and collections. 3. Worthen Collection. I. Title. II. Series. Editor: Mary Clockner Cover: Sandra Stecyk Printed by the authority of the State of Illinois/1982/2500 II I IHOI'.MAII '.I 'II Of.ir.AI MIHVI y '> 300 1 00003 5216 TYPE AND FIGURED FOSSILS IN THE WORTHEN COLLECTION AT THE ILLINOIS STATE GEOLOGICAL SURVEY Lois S. Kent | CIRCULAR 524 1982 ILLINOIS STATE GEOLOGICAL SURVEY Robert E. Bergstrom, Acting Chief Natural Resources Building, 615 East Peabody Drive, Champaign, IL 61820 TYPE AND FIGURED FOSSILS IN THE WORTHEN COLLECTION AT THE ILLINOIS STATE GEOLOGICAL SURVEY CONTENTS Acknowledgments 2 Introduction 2 Organization of the catalog 7 Notes 8 References 8 Fossil catalog 13 ABSTRACT This catalog lists all type and figured specimens of fossils in the part of the "Worthen Collection" now housed at the Illinois State Geological Survey in Champaign, Illinois. -

MYRIAPODS 767 Volume 2 (M-Z), Pp
In: R. Singer, (ed.), 1999. Encyclopedia of Paleontology, MYRIAPODS 767 volume 2 (M-Z), pp. 767-775. Fitzroy Dearborn, London. MYRIAPODS JVlyriapods are many-legged, terrestrial arthropods whose bodies groups, the Trilobita, Chelicerata, Crustacea, and the Uniramia, the are divided into two major parts, a head and a trunk. The head last consisting of the Myriapoda, Hexapoda, and Onychophora (vel- bears a single pair of antennae, highly differentiated mandibles (or vet worms). However, subsequent structural and molecular evidence jaws), and at least one pair of maxillary mouthparts; the trunk indicates that there are several characters uniting major arthropod region consists of similar "metameres," each of which is a func- taxa. Moreover, paleobiologic, embryologie, and other evidence tional segment that bears one or two pairs of appendages. Gas demonstrates that myriapods and hexapods are fiindamentally exchange is accomplished by tracheae•a branching network of polyramous, having two major articulating appendages per embry- specialized tubules•although small forms respire through the ological body segment, like other arthropods. body wall. Malpighian organs are used for excretion, and eyes con- A fourth proposal (Figure ID) suggests that myriapods are sist of clusters of simple, unintegrated, light-sensitive elements an ancient, basal arthropod lineage, and that the Hexapoda that are termed ommatidia. These major features collectively char- emerged as an independent, relatively recent clade from a rather acterize the five major myriapod clades: Diplopoda (millipeds), terminal crustacean lineage, perhaps the Malacostraca, which con- Chilopoda (centipeds), Pauropoda (pauropods), Symphyla (sym- tains lobsters and crabs (Ballard et al. 1992). Because few crusta- phylans), and Arthropleurida (arthropleurids). Other features cean taxa were examined in this analysis, and due to the Cambrian indicate differences among these clades. -
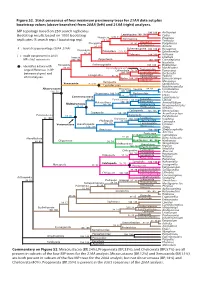
Figure S2. Strict Consensus of Four Maximum Parsimony Trees for 21AA Data Set Plus Bootstrap Values (Above Branches) from 20AA (Left) and 21AA (Right) Analyses
Figure S2. Strict consensus of four maximum parsimony trees for 21AA data set plus bootstrap values (above branches) from 20AA (left) and 21AA (right) analyses. MP topology based on 250 search replicates. 100 100 Antheraea Bootstrap results based on 1000 bootstrap Lepidoptera 100 100 Cydia Neoptera [23] 44 Dermaptera Prodoxus replicates (5 search reps / bootstrap rep). 68 66 Forficula Blattodea Pterygota 92 96 Periplaneta Orthoptera 96 97 Acheta # : bootstrap percentage (20AA 21AA) Ephemeroptera Dicondylia 100 100 Hexagenia Paleoptera [37] 52 Ephemerella 98 98 Odonata 100 100 Ischnura [ ] : node not present in 20AA Insecta Libellula MP strict consensus 100 100 Zygentoma 100 100 Ctenolepisma Nicoletia Archaeognatha : Identifies 6 taxa with Hexapoda 100 100 Pedetontus 89 86 Machiloides Entomobryomorpha Tomoceridae large differences in BP Collembola 70 76 Tomocerus between degen1 and 100 100 Entomobryidae Orchesella Entognatha 82 82 Poduridae Podura 20AA analyses. Diplura 100 100 Campodeidae Japygidae Eumesocampa Remipedia Metajapyx Xenocarida [31] 51 Speleonectes Cephalocarida Hutchinsoniella Altocrustacea Thoracica Sessilia 88 83 Semibalanus [14] 31 100 100 Chthamalus Thecostraca 100 100 Pedunculata Lepas Communostraca Rhizocephala Loxothylacus 96 89 Eumalacostraca 84 85 Eucarida Libinia Malacostraca 100 100 Peracarida Armadillidium Multicrustacea 100 100 Hoplocarida 69 84 Neogonodactylus Phyllocarida Nebalia Cyclopoida 100 100 Mesocyclops Copepoda 100 100 Acanthocyclops Pancrustacea 65 82 Calanoida Eurytemora 96 100 Diplostraca 100 100 -

Exploring Phylogenomic Relationships Within Myriapoda: Should High Matrix Occupancy Be the Goal?
bioRxiv preprint doi: https://doi.org/10.1101/030973; this version posted November 9, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Exploring phylogenomic relationships within Myriapoda: should high matrix occupancy be the goal? ROSA FERNÁNDEZ1, GREGORY D. EDGECOMBE2 AND GONZALO GIRIBET1 1Museum of Comparative Zoology & Department of Organismic and Evolutionary Biology, Harvard University, 26 Oxford Street, Cambridge, MA 02138, USA 2Department of Earth Sciences, The Natural History Museum, Cromwell Road, London SW7 5BD, UK 1 bioRxiv preprint doi: https://doi.org/10.1101/030973; this version posted November 9, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract.—Myriapods are one of the dominant terrestrial arthropod groups including the diverse and familiar centipedes and millipedes. Although molecular evidence has shown that Myriapoda is monophyletic, its internal phylogeny remains contentious and understudied, especially when compared to those of Chelicerata and Hexapoda. Until now, efforts have focused on taxon sampling (e.g., by including a handful of genes in many species) or on maximizing matrix occupancy (e.g., by including hundreds or thousands of genes in just a few species), but a phylogeny maximizing sampling at both levels remains elusive. In this study, we analyzed forty Illumina transcriptomes representing three myriapod classes (Diplopoda, Chilopoda and Symphyla); twenty-five transcriptomes were newly sequenced to maximize representation at the ordinal level in Diplopoda and at the family level in Chilopoda. -

A New Species of Illacme Cook & Loomis, 1928
A peer-reviewed open-access journal ZooKeys 626: 1–43A new (2016) species of Illacme Cook and Loomis, 1928 from Sequoia National Park... 1 doi: 10.3897/zookeys.626.9681 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research A new species of Illacme Cook & Loomis, 1928 from Sequoia National Park, California, with a world catalog of the Siphonorhinidae (Diplopoda, Siphonophorida) Paul E. Marek1, Jean K. Krejca2, William A. Shear3 1 Virginia Polytechnic Institute and State University, Department of Entomology, Price Hall, Blacksburg, Virginia, USA 2 Zara Environmental LLC, 1707 W FM 1626, Manchaca, Texas, USA 3 Hampden-Sydney College, Department of Biology, Gilmer Hall, Hampden-Sydney, Virginia, USA Corresponding author: Paul E. Marek ([email protected]) Academic editor: R. Mesibov | Received 25 July 2016 | Accepted 19 September 2016 | Published 20 October 2016 http://zoobank.org/36E16503-BC2B-4D92-982E-FC2088094C93 Citation: Marek PE, Krejca JK, Shear WA (2016) A new species of Illacme Cook & Loomis, 1928 from Sequoia National Park, California, with a world catalog of the Siphonorhinidae (Diplopoda, Siphonophorida). ZooKeys 626: 1–43. doi: 10.3897/zookeys.626.9681 Abstract Members of the family Siphonorhinidae Cook, 1895 are thread-like eyeless millipedes that possess an astounding number of legs, including one individual with 750. Due to their cryptic lifestyle, rarity in natural history collections, and sporadic study over the last century, the family has an unclear phylogenetic placement, and intrafamilial relationships remain unknown. Here we report the discovery of a second spe- cies of Illacme, a millipede genus notable for possessing the greatest number of legs of any known animal on the planet. -

Ordinal-Level Phylogenomics of the Arthropod Class
Ordinal-Level Phylogenomics of the Arthropod Class Diplopoda (Millipedes) Based on an Analysis of 221 Nuclear Protein-Coding Loci Generated Using Next- Generation Sequence Analyses Michael S. Brewer1,2*, Jason E. Bond3 1 Department of Environmental Science, Policy, and Management, University of California Berkeley, Berkeley, California, United States of America, 2 Department of Biology, East Carolina University, Greenville, North Carolina, United States of America, 3 Department of Biological Sciences and Auburn University Museum of Natural History, Auburn University, Auburn, Alabama, United States of America Abstract Background: The ancient and diverse, yet understudied arthropod class Diplopoda, the millipedes, has a muddled taxonomic history. Despite having a cosmopolitan distribution and a number of unique and interesting characteristics, the group has received relatively little attention; interest in millipede systematics is low compared to taxa of comparable diversity. The existing classification of the group comprises 16 orders. Past attempts to reconstruct millipede phylogenies have suffered from a paucity of characters and included too few taxa to confidently resolve relationships and make formal nomenclatural changes. Herein, we reconstruct an ordinal-level phylogeny for the class Diplopoda using the largest character set ever assembled for the group. Methods: Transcriptomic sequences were obtained from exemplar taxa representing much of the diversity of millipede orders using second-generation (i.e., next-generation or high-throughput) sequencing. These data were subject to rigorous orthology selection and phylogenetic dataset optimization and then used to reconstruct phylogenies employing Bayesian inference and maximum likelihood optimality criteria. Ancestral reconstructions of sperm transfer appendage development (gonopods), presence of lateral defense secretion pores (ozopores), and presence of spinnerets were considered.