RAD-AID Global Curriculum for Interventional Radiology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Eliminate Varicose Veins with No Surgery and Scars
CARIBBEAN VASCULAR & VEIN CLINIC www.cvctt.com Now you can show immediate improvement on your legs by eliminating varicose veins with EVLT®. This 45-minute laser procedure requires no general anesthesia or hospitalization, meaning no painful surgery or downtime. Deciding to get the safe and effective EVLT® is a choice everyone will notice. Other Options Offered for Veins • Sclerotherapy • Subfascial Endoscopic Perforator Ligation (SEPS) • COMPASS protocol of ultrasound- guided • Conventional Open sclerotherapy. Surgery (High or CARIBBEAN Sapheno-femoral Ligation • Ambulatory Phlebectomy and Stab Avulsion) VASCULAR & VEIN CLINIC For Other Vascular Disorders • Diabetic feet • Peripheral Vascular disease (poor circulation) • Aneurysms • Dialysis Access for Renal Failure • Carotid interventions for Strokes • 45-minute CARIBBEAN procedure • No scarring VASCULAR & VEIN • No general CLINIC Eliminate anesthesia or hospitalization Varicose Veins • Immediate return to your daily St. Clair Medical Centre, with no surgery routine 18 Elizabeth Street, St. Clair, Port of Spain, Trinidad, West Indies Search: CARIBBEAN VASCULAR & VEIN CLINIC T: (868) 622 9665 • F: (868) 622 9665 and scars E: [email protected] CARIBBEAN VASCULAR & VEIN CLINIC www.cvctt.com About EVLT® EVLT® is a quick, minimally invasive laser procedure that leaves no scar and can be performed in the vascular clinic under local anesthesia. The treatment takes less than one hour. What does EVLT® stand for? Here’s what to expect EndoVenous Laser Treatment • Your doctor uses ultrasound to map out your vein. Varicose Vein Treatment How does the procedure actually work? The images below illustrate varicose vein pictures from • Local anesthetic is applied. A laser fiber is fired inside and along the length of your before and after EVLT® treatment. -

Varicose Vein and Venous Insufficiency Treatments
Medica Policy No. III-SUR.26 UTILIZATION MANAGEMENT POLICY TITLE: VARICOSE VEIN AND VENOUS INSUFFICIENCY TREATMENTS EFFECTIVE DATE: January 18, 2021 This policy was developed with input from specialists in general surgery, vascular surgery and interventional radiology, and endorsed by the Medical Policy Committee. IMPORTANT INFORMATION – PLEASE READ BEFORE USING THIS POLICY These services may or may not be covered by all Medica plans. Please refer to the member’s plan document for specific coverage information. If there is a difference between this general information and the member’s plan document, the member’s plan document will be used to determine coverage. With respect to Medicare and Minnesota Health Care Programs, this policy will apply unless these programs require different coverage. Members may contact Medica Customer Service at the phone number listed on their member identification card to discuss their benefits more specifically. Providers with questions about this Medica utilization management policy may call the Medica Provider Service Center toll-free at 1-800-458-5512. Medica utilization management policies are not medical advice. Members should consult with appropriate health care providers to obtain needed medical advice, care and treatment. PURPOSE To promote consistency between utilization management reviewers by providing the criteria that determines the medical necessity. BACKGROUND Definitions: A. Cyanoacrylate adhesive closure of symptomatic varicose veins is a minimally invasive procedure that uses a specially formulated medical adhesive (glue) that is injected into the vein using the VenaSeal™ closure system. The cyanoacrylate adhesive permanently closes the diseased vein. B. Duplex ultrasonography/Doppler ultrasound are two imaging modalities done sequentially to outline anatomical structure of blood vessels(duplex) and to detect flow, direction of flow and flow velocity of the blood through vessels. -

Selected Treatments for Varicose Veins Final Evidence Report
Selected treatments for varicose veins Final evidence report April 14, 2017 Health Technology Assessment Program (HTA) Washington State Health Care Authority PO Box 42712 Olympia, WA 98504-2712 (360) 725-5126 www.hca.wa.gov/about-hca/health-technology-assessment [email protected] Selected Treatments for Varicose Veins A Health Technology Assessment Prepared for Washington State Health Care Authority Final REPORT April 14, 2017 Acknowledgement This report was prepared by: Hayes, Inc. 157 S. Broad Street Suite 200 Lansdale, PA 19446 P: 215.855.0615 F: 215.855.5218 This report is intended to provide research assistance and general information only. It is not intended to be used as the sole basis for determining coverage policy or defining treatment protocols or medical modalities, nor should it be construed as providing medical advice regarding treatment of an individual’s specific case. Any decision regarding claims eligibility or benefits, or acquisition or use of a health technology is solely within the discretion of your organization. Hayes, Inc. assumes no responsibility or liability for such decisions. Hayes employees and contractors do not have material, professional, familial, or financial affiliations that create actual or potential conflicts of interest related to the preparation of this report. WA – Health Technology Assessment April 14, 2017 Table of Contents EVIDENCE SUMMARY ......................................................................................................................... 1 Summary of Clinical Background -

Imagingadvances
ImagingAdvances VOLUME 5 ISSUE 1 Spring 2013 New Facility Supports More In-Office Procedures by VIVA’s Highly Trained Vascular Surgeons Local Physicians Consult with RAF When Imaging is Vital to Patient Care Diagnostic radiologists are best known for their behind-the-scenes interpretation of medical imaging test results. Yet their collaboration with other medical specialists is equally critical to patient care. David L. Glasser, MD, president of Radiologic Associates of Fredericksburg (RAF), said he and the practice’s other board-certified, fellowship-trained diagnostic radiologists spend a significant part of each day discussing cases and medical imaging tests with patients’ physicians. Typical cases include: • Answering an orthopedic surgeon’s questions about a patient’s imaging tests before surgery to repair a torn meniscus. • Showing an oncologist how a patient’s lymph nodes and tumors are responding to cancer treatment. Dr. D’Addio (right) views real-time medical images to guide a vascular surgery procedure at VIVA. He is assisted by Medical Assistant Stephanie Finley. • Discussing test findings with a patient’s primary care physician When local vascular surgeon Victor J. D’Addio, MD, announced he was joining Radiologic to aid in a diagnosis. Associates of Fredericksburg (RAF) in 2009, some of his patients and medical colleagues Diagnostic Radiology continued page 3 were perplexed. Was Dr. D’Addio switching medical specialties? The answer was “no.” Dr. D’Addio moved his vascular surgery practice to RAF’s interventional radiology and vascular program, Virginia Interventional & Vascular Associates (VIVA), because the two specialties complement each other. In fact, several other radiology groups with interventional radiology sub-specialists in the United States have vascular surgeons as part of their staff. -

Ambulatory Phlebectomy INFORMED CONSENT
Ambulatory Phlebectomy INFORMED CONSENT INTRODUCTION: Ambulatory Phlebectomy is a procedure used to remove varicose veins that are near the surface. This procedure is done under local anesthesia. This treatment is used to remove larger veins that are close to the surface. Small stab incisions are made in the skin and the veins are pulled out of these incisions with a small hook. There will be many small incisions in the skin. These will incisions will leave small scars. The incisions may be left open, closed with small pieces of tape or occasionally sutured closed. Occasionally a slightly larger incision will be needed to remove larger veins or in the presence of scar tissue making the dissection more tedious. The pain is usually minor and little scarring is anticipated. A relatively short recovery following the procedure can be expected. The veins being treated are generally removed in one treatment. Additional veins can develop in the areas treated but the vessels removed will not come back. PROCEDURE DESCRIPTION: On the day of your Ambulatory Phlebectomy procedure, staff at The re*be Skin Clinic will answer any last minute questions you might have, and prepare you for the procedure. The skin will be marked to show the location of the veins to be removed. These marks can remain visible on you skin for about 1 week rarely more than that. Hair in the treatment areas may be clipped and the skin will be cleansed. You will be positioned on a power procedure table. Medicated ointment may be applied to your skin to temporarily anesthetize the area being treated. -

Surgical and Interventional Radiology Antimicrobial Prophylaxis – Adult/Pediatric – Inpatient/Ambulatory/Emergency Department Clinical Practice Guideline
Surgical and Interventional Radiology Antimicrobial Prophylaxis – Adult/Pediatric – Inpatient/Ambulatory/Emergency Department Clinical Practice Guideline Table of Contents EXECUTIVE SUMMARY ........................................................................................................... 3 SCOPE ...................................................................................................................................... 3 METHODOLOGY ...................................................................................................................... 4 DEFINITIONS ............................................................................................................................ 4 INTRODUCTION ....................................................................................................................... 4 TABLE 1. SURGICAL PROPHYLAXIS ANTIMICROBIAL DOSING ......................................... 6 TABLE 2. REDOSING INTERVALS .......................................................................................... 7 TABLE 3. RECOMMENDED ADMINISTRATION TIMES .......................................................... 8 TABLE 4. SURGICAL PROPHYLAXIS ANTIMICROBIAL SELECTION AT UWHC ................. 9 TABLE 5. INTERVENTIONAL RADIOLOGY PROPHYLAXIS ANTIMICROBIAL SELECTION .............................................................................................................................16 RECOMMENDATIONS .............................................................................................................20 -

Varicose Veins
Journal of Radiology Nursing 38 (2019) 150e154 Contents lists available at ScienceDirect Journal of Radiology Nursing journal homepage: www.sciencedirect.com/journal/ journal-of-radiology-nursing Varicose Veins * Matthew Wang, MD a, Ashwani K. Sharma, MD b, a Department of Imaging Sciences, University of Rochester, Rochester, NY, USA b Division of Interventional Radiology, Department of Imaging Sciences, University of Rochester, Rochester, NY, USA abstract Keywords: Varicose veins are enlarged, tortuous veins that are commonly found in the lower extremities, affecting Varicose veins people over a wide age range with increasing prevalence with age. This pathology is typically a benign Phlebectomy process with complications that can decrease a person's quality of life and lead to potentially life- Sclerotherapy threatening complications. There are surgical, endovascular, and chemical treatments which improve Laser ablation quality of life and decrease secondary complications of varicose veins. This review article will discuss the Radiofrequency ablation technical aspects of these procedures, potential and rare complications, preprocedural and post- procedural considerations, and overall comparison of technique efficacy. © 2019 Association for Radiologic & Imaging Nursing. Published by Elsevier Inc. All rights reserved. Background veins and the tortuous appearance of varicose veins. Metabolic waste products build up in these engorged vessels and inflamma- Varicose veins (Figure 1) are enlarged, tortuous veins that are tion precipitates pain, burning, itching, cramping, and edema commonly found in the lower extremities, affecting people over a (Bergan et al., 2006). wide age range with increasing prevalence with age. These veins Common complications of varicose veins include debility and can cause symptoms of painful swelling of the lower legs, which decreased quality of life and function from the pain and inflam- may be associated with pruritus and discoloration of the skin. -

SPP PW R4.Indd
Bajaj Allianz General Insurance Company Limited CIN: U66010PN2000PLC015329 Issuing Office : UIN: IRDA/NL-HLT/BAGI/P-H/V.I/21/13-14 SURGICAL PROTECTION PLAN POLICY DOCUMENT Surgical Protection Plan has 2 Sections 1. Section I: Surgical Benefit Cover (with 11 plans) 2. Section II: Add On covers (Optional) A. Hospital Cash Daily Allowance B. Critical Illness Cover C. Personal Accident Cover Types of Policy • Individual Surgical Protection Plan Policy • Group Surgical Protection Plan Policy Policy period This is an annual policy Preamble Our agreement to insure You is based on Your Proposal to Us, which is the basis of this agreement, and Your payment of the premium. This Policy records the entire agreement between us and sets out what We insure, how We insure it, and what We expect of You and what You can expect of Us. Scope of cover The Company hereby agrees to pay in respect of an admissible claim, any or all of the following covers subject to the Sum Insured, limits, terms, conditions and definitions, exclusions contained or otherwise expressed in this Policy. Section I : Surgical Benefit Cover Coverage 1. If You are Hospitalised on the advice of a Doctor because of Illness or Accidental Bodily Injury sustained or contracted during the Policy Period and undergo a Surgical Procedure which is listed in Annexure 1 of this policy document, then We will pay You a benefit amount as per the grade of the Surgery and the Sum Insured shown on the Schedule. 2. Specific Exclusions Applicable for Surgical Benefit Cover We will not pay for claims arising out of or howsoever connected to the following: i. -

VIR PERIPROCEDURAL ANTICOAGULATION GUIDELINES Procedure Category LOW RISK MODERATE RISK HIGH RISK
VIR PERIPROCEDURAL ANTICOAGULATION GUIDELINES Procedure Category LOW RISK MODERATE RISK HIGH RISK * If on blood thinner or Coumadin held for Angiography (arterial intervention up to 7fr procedure, check INR prior to the procedure* Sheath) TIPS Non-Tunneled venous access (PICC, Central line, Tunneled PICC, temp HD Cath) Thrombolysis / Lysis Check Renal Biopsy Central venous line removal, Tunneled venous line removal Venogram (groin puncture) Thermal Ablation of Tumor Venography (foot or arm access) Venous interventions/ Thrombolysis Nephrotomy tube placement Catheter exchange (biliary, nephrostomy, Mapping Angiogram / Y90 / DEB / Biliary interventions *new access* (PTC & abscess drain) Chemoembolization Biliary Stent) Tunneled venous access (Port Placement, Paracentesis Perm Cath, PICC) Lung Biopsy Thyroid Biopsy Port Removal Spleen Biopsy Superficial Biopsy (lymph node) Transjugular Liver or Kidney Biopsy AAA Repair Superficial abscess drainage or aspiration Spine Procedures (vertebroplasty, vertebral Abscess Tube Check BRTO augmenation, sacroplasty) Percutaneous drain placement, Chest Tube placement Abdomen/Pelvis Uterine fibroid embolization Biopsy (Liver, Deep Lymph node, Spine, Other G-Tube exchange/ replacement organs) Venous Access (trans-lumbar IVC Catheter) Venous Ablation (RF/ Laser) Thoracentesis Venous Sclerotherapy Pulmonary AVM Embolization Ambulatory Phlebectomy Pleurex (Abdominal & Thoracic) Vulva Sclero Denver Shunt IVC Filter Placement Cholecystomstomy Fistula Declot G-Tube Placement AV Fistulagram / plasty Ureteral Stent -
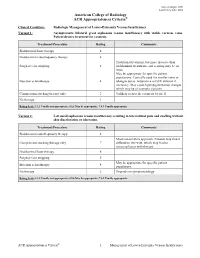
Radiologic Management of Lower-Extremity Venous Insufficiency Variant 1: Asymptomatic Bilateral Great Saphenous Venous Insufficiency with Visible Varicose Veins
Date of origin: 2009 Last review date: 2012 American College of Radiology ® ACR Appropriateness Criteria Clinical Condition: Radiologic Management of Lower-Extremity Venous Insufficiency Variant 1: Asymptomatic bilateral great saphenous venous insufficiency with visible varicose veins. Patient desires treatment for cosmesis. Treatment/Procedure Rating Comments Endoluminal laser therapy 8 Endoluminal radiofrequency therapy 8 Traditional treatment, but more invasive than Surgical vein stripping 4 endoluminal treatments, and scarring may be an issue. May be appropriate for specific patient populations. Typically used for smaller veins or Injection sclerotherapy 4 telangiectasias. Adjunctive to GSV ablation if necessary. May cause hyperpigmentation changes which may be of cosmetic concern. Compression stocking therapy only 2 Unlikely to provide cosmesis by itself. No therapy 1 Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Variant 2: Left small saphenous venous insufficiency resulting in intermittent pain and swelling without skin discoloration or ulceration. Treatment/Procedure Rating Comments Endoluminal radiofrequency therapy 8 Most conservative approach. Patients may find it Compression stocking therapy only 7 difficult to live with, which may lead to noncompliance with therapy. Endoluminal laser therapy 8 Surgical vein stripping 5 May be appropriate for specific patient Injection sclerotherapy 4 populations. No therapy 2 Depends on symptomatology. Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate ACR Appropriateness Criteria® 1 Management of Lower-Extremity Venous Insufficiency Clinical Condition: Radiologic Management of Lower-Extremity Venous Insufficiency Variant 3: Left great saphenous venous insufficiency with associated lower leg skin ulceration. -

A Review of Minimally Invasive Techniques for the Treatment of Lower Extremity Varicose Veins
A Review of Minimally Invasive Techniques for the Treatment of Lower Extremity Varicose Veins Poster No.: R-0103 Congress: 2015 ASM Type: Educational Exhibit Authors: N. Burns, A. Galea, S. Nadkarni; Perth/AU Keywords: Varices, Venous access, Laser, Ablation procedures, Ultrasound, Veins / Vena cava, Vascular, Interventional vascular DOI: 10.1594/ranzcr2015/R-0103 Any information contained in this pdf file is automatically generated from digital material submitted to EPOS by third parties in the form of scientific presentations. References to any names, marks, products, or services of third parties or hypertext links to third- party sites or information are provided solely as a convenience to you and do not in any way constitute or imply RANZCR's endorsement, sponsorship or recommendation of the third party, information, product or service. RANZCR is not responsible for the content of these pages and does not make any representations regarding the content or accuracy of material in this file. As per copyright regulations, any unauthorised use of the material or parts thereof as well as commercial reproduction or multiple distribution by any traditional or electronically based reproduction/publication method ist strictly prohibited. You agree to defend, indemnify, and hold RANZCR harmless from and against any and all claims, damages, costs, and expenses, including attorneys' fees, arising from or related to your use of these pages. Please note: Links to movies, .ppt slideshows, .doc documents and any other multimedia files are not available in the pdf version of presentations. Page 1 of 15 Learning objectives In this educational poster we will provide an outline of ultrasound-guided minimally invasive procedures combined with a minimally invasive surgical procedure for the treatment of varicose veins in an outpatient setting. -

Abstract Book
CONTROVERSES ET ACTUALITÉS EN CHIRURGIE VASCULAIRE CONTROVERSIES & UPDATES Chairman IN VASCULAR SURGERY Jean-Pierre Becquemin Co-chairman Venous Session JANUARY 19-21 2017 Jean-Luc Gérard MARRIOTT RIVE GAUCHE & CONFERENCE CENTER Co-chairman Hemodialysis angioaccesses Session PARIS, FRANCE www.cacvs.org Eric Chemla Scientific Committee Eric Allaire Jean-Marc Alsac Michel Bartoli Pierre Bourquelot Ludovic Canaud Xavier Chaufour Nick Cheshire Frédéric Cochennec Eric Ducasse Hans-Henning Eckstein Michel Ferdani Jean-François Garbé ABSTRACT Yann Gouëffic Christos Liapis Ian Loftus Martin Malina Armando Mansilha BOOK Maxime Sibé Hence Verhagen Frank Vermassen Fabio Verzini Venous Scientific Committee Claudine Hamel-Desnos Lowell Kabnick Michel Perrin Thomas Proebstle Chairman Pr Jean-Pierre BECQUEMIN MD, Professor of Vascular Surgery, IVPE, Champigny, France Table of contents Faculty authors Co-chairman Venous Session CONTROVERSIES & UPDATES IN VASCULAR SURGERY VASCULAR PROGRAM VENOUS PROGRAM Dr Jean-Luc GÉRARD AMIN Ali ...................................... 52 AMIN Ali ...................................... 70 MD, Paris, France BICKNELL Colin .................................. 6 BONE Carlos.................................... 74 BULBULIA Richard ............................... 22 DROC Ionel .................................... 84 Co-chairman Hemodialysis THURSDAY JANUARY 19 DONAS KOSTANTINOS ........................... 20 EKLÖF Bo ................................... 68, 77 angioaccesses Session FANNELI Fabrizio................................