Synthesis and Characterization of Fe3o4 Nanoparticles with Perspectives in Biomedical Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Canted Ferrimagnetism and Giant Coercivity in the Non-Stoichiometric
Canted ferrimagnetism and giant coercivity in the non-stoichiometric double perovskite La2Ni1.19Os0.81O6 Hai L. Feng1, Manfred Reehuis2, Peter Adler1, Zhiwei Hu1, Michael Nicklas1, Andreas Hoser2, Shih-Chang Weng3, Claudia Felser1, Martin Jansen1 1Max Planck Institute for Chemical Physics of Solids, Dresden, D-01187, Germany 2Helmholtz-Zentrum Berlin für Materialien und Energie, Berlin, D-14109, Germany 3National Synchrotron Radiation Research Center (NSRRC), Hsinchu, 30076, Taiwan Abstract: The non-stoichiometric double perovskite oxide La2Ni1.19Os0.81O6 was synthesized by solid state reaction and its crystal and magnetic structures were investigated by powder x-ray and neutron diffraction. La2Ni1.19Os0.81O6 crystallizes in the monoclinic double perovskite structure (general formula A2BB’O6) with space group P21/n, where the B site is fully occupied by Ni and the B’ site by 19 % Ni and 81 % Os atoms. Using x-ray absorption spectroscopy an Os4.5+ oxidation state was established, suggesting presence of about 50 % 5+ 3 4+ 4 paramagnetic Os (5d , S = 3/2) and 50 % non-magnetic Os (5d , Jeff = 0) ions at the B’ sites. Magnetization and neutron diffraction measurements on La2Ni1.19Os0.81O6 provide evidence for a ferrimagnetic transition at 125 K. The analysis of the neutron data suggests a canted ferrimagnetic spin structure with collinear Ni2+ spin chains extending along the c axis but a non-collinear spin alignment within the ab plane. The magnetization curve of La2Ni1.19Os0.81O6 features a hysteresis with a very high coercive field, HC = 41 kOe, at T = 5 K, which is explained in terms of large magnetocrystalline anisotropy due to the presence of Os ions together with atomic disorder. -

Magnetism, Magnetic Properties, Magnetochemistry
Magnetism, Magnetic Properties, Magnetochemistry 1 Magnetism All matter is electronic Positive/negative charges - bound by Coulombic forces Result of electric field E between charges, electric dipole Electric and magnetic fields = the electromagnetic interaction (Oersted, Maxwell) Electric field = electric +/ charges, electric dipole Magnetic field ??No source?? No magnetic charges, N-S No magnetic monopole Magnetic field = motion of electric charges (electric current, atomic motions) Magnetic dipole – magnetic moment = i A [A m2] 2 Electromagnetic Fields 3 Magnetism Magnetic field = motion of electric charges • Macro - electric current • Micro - spin + orbital momentum Ampère 1822 Poisson model Magnetic dipole – magnetic (dipole) moment [A m2] i A 4 Ampere model Magnetism Microscopic explanation of source of magnetism = Fundamental quantum magnets Unpaired electrons = spins (Bohr 1913) Atomic building blocks (protons, neutrons and electrons = fermions) possess an intrinsic magnetic moment Relativistic quantum theory (P. Dirac 1928) SPIN (quantum property ~ rotation of charged particles) Spin (½ for all fermions) gives rise to a magnetic moment 5 Atomic Motions of Electric Charges The origins for the magnetic moment of a free atom Motions of Electric Charges: 1) The spins of the electrons S. Unpaired spins give a paramagnetic contribution. Paired spins give a diamagnetic contribution. 2) The orbital angular momentum L of the electrons about the nucleus, degenerate orbitals, paramagnetic contribution. The change in the orbital moment -

Chapter 6 Antiferromagnetism and Other Magnetic Ordeer
Chapter 6 Antiferromagnetism and Other Magnetic Ordeer 6.1 Mean Field Theory of Antiferromagnetism 6.2 Ferrimagnets 6.3 Frustration 6.4 Amorphous Magnets 6.5 Spin Glasses 6.6 Magnetic Model Compounds TCD February 2007 1 1 Molecular Field Theory of Antiferromagnetism 2 equal and oppositely-directed magnetic sublattices 2 Weiss coefficients to represent inter- and intra-sublattice interactions. HAi = n’WMA + nWMB +H HBi = nWMA + n’WMB +H Magnetization of each sublattice is represented by a Brillouin function, and each falls to zero at the critical temperature TN (Néel temperature) Sublattice magnetisation Sublattice magnetisation for antiferromagnet TCD February 2007 2 Above TN The condition for the appearance of spontaneous sublattice magnetization is that these equations have a nonzero solution in zero applied field Curie Weiss ! C = 2C’, P = C’(n’W + nW) TCD February 2007 3 The antiferromagnetic axis along which the sublattice magnetizations lie is determined by magnetocrystalline anisotropy Response below TN depends on the direction of H relative to this axis. No shape anisotropy (no demagnetizing field) TCD February 2007 4 Spin Flop Occurs at Hsf when energies of paralell and perpendicular configurations are equal: HK is the effective anisotropy field i 1/2 This reduces to Hsf = 2(HKH ) for T << TN Spin Waves General: " n h q ~ q ! M and specific heat ~ Tq/n Antiferromagnet: " h q ~ q ! M and specific heat ~ Tq TCD February 2007 5 2 Ferrimagnetism Antiferromagnet with 2 unequal sublattices ! YIG (Y3Fe5O12) Iron occupies 2 crystallographic sites one octahedral (16a) & one tetrahedral (24d) with O ! Magnetite(Fe3O4) Iron again occupies 2 crystallographic sites one tetrahedral (8a – A site) & one octahedral (16d – B site) 3 Weiss Coefficients to account for inter- and intra-sublattice interaction TCD February 2007 6 Below TN, magnetisation of each sublattice is zero. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

Magnetism in Transition Metal Complexes
Magnetism for Chemists I. Introduction to Magnetism II. Survey of Magnetic Behavior III. Van Vleck’s Equation III. Applications A. Complexed ions and SOC B. Inter-Atomic Magnetic “Exchange” Interactions © 2012, K.S. Suslick Magnetism Intro 1. Magnetic properties depend on # of unpaired e- and how they interact with one another. 2. Magnetic susceptibility measures ease of alignment of electron spins in an external magnetic field . 3. Magnetic response of e- to an external magnetic field ~ 1000 times that of even the most magnetic nuclei. 4. Best definition of a magnet: a solid in which more electrons point in one direction than in any other direction © 2012, K.S. Suslick 1 Uses of Magnetic Susceptibility 1. Determine # of unpaired e- 2. Magnitude of Spin-Orbit Coupling. 3. Thermal populations of low lying excited states (e.g., spin-crossover complexes). 4. Intra- and Inter- Molecular magnetic exchange interactions. © 2012, K.S. Suslick Response to a Magnetic Field • For a given Hexternal, the magnetic field in the material is B B = Magnetic Induction (tesla) inside the material current I • Magnetic susceptibility, (dimensionless) B > 0 measures the vacuum = 0 material response < 0 relative to a vacuum. H © 2012, K.S. Suslick 2 Magnetic field definitions B – magnetic induction Two quantities H – magnetic intensity describing a magnetic field (Système Internationale, SI) In vacuum: B = µ0H -7 -2 µ0 = 4π · 10 N A - the permeability of free space (the permeability constant) B = H (cgs: centimeter, gram, second) © 2012, K.S. Suslick Magnetism: Definitions The magnetic field inside a substance differs from the free- space value of the applied field: → → → H = H0 + ∆H inside sample applied field shielding/deshielding due to induced internal field Usually, this equation is rewritten as (physicists use B for H): → → → B = H0 + 4 π M magnetic induction magnetization (mag. -

Magnetic Materials: Hysteresis
Magnetic Materials: Hysteresis Ferromagnetic and ferrimagnetic materials have non-linear initial magnetisation curves (i.e. the dotted lines in figure 7), as the changing magnetisation with applied field is due to a change in the magnetic domain structure. These materials also show hysteresis and the magnetisation does not return to zero after the application of a magnetic field. Figure 7 shows a typical hysteresis loop; the two loops represent the same data, however, the blue loop is the polarisation (J = µoM = B-µoH) and the red loop is the induction, both plotted against the applied field. Figure 7: A typical hysteresis loop for a ferro- or ferri- magnetic material. Illustrated in the first quadrant of the loop is the initial magnetisation curve (dotted line), which shows the increase in polarisation (and induction) on the application of a field to an unmagnetised sample. In the first quadrant the polarisation and applied field are both positive, i.e. they are in the same direction. The polarisation increases initially by the growth of favourably oriented domains, which will be magnetised in the easy direction of the crystal. When the polarisation can increase no further by the growth of domains, the direction of magnetisation of the domains then rotates away from the easy axis to align with the field. When all of the domains have fully aligned with the applied field saturation is reached and the polarisation can increase no further. If the field is removed the polarisation returns along the solid red line to the y-axis (i.e. H=0), and the domains will return to their easy direction of magnetisation, resulting in a decrease in polarisation. -

Condensed Matter Option MAGNETISM Handout 1
Condensed Matter Option MAGNETISM Handout 1 Hilary 2014 Radu Coldea http://www2.physics.ox.ac.uk/students/course-materials/c3-condensed-matter-major-option Syllabus The lecture course on Magnetism in Condensed Matter Physics will be given in 7 lectures broken up into three parts as follows: 1. Isolated Ions Magnetic properties become particularly simple if we are able to ignore the interactions between ions. In this case we are able to treat the ions as effectively \isolated" and can discuss diamagnetism and paramagnetism. For the latter phenomenon we revise the derivation of the Brillouin function outlined in the third-year course. Ions in a solid interact with the crystal field and this strongly affects their properties, which can be probed experimentally using magnetic resonance (in particular ESR and NMR). 2. Interactions Now we turn on the interactions! I will discuss what sort of magnetic interactions there might be, including dipolar interactions and the different types of exchange interaction. The interactions lead to various types of ordered magnetic structures which can be measured using neutron diffraction. I will then discuss the mean-field Weiss model of ferromagnetism, antiferromagnetism and ferrimagnetism and also consider the magnetism of metals. 3. Symmetry breaking The concept of broken symmetry is at the heart of condensed matter physics. These lectures aim to explain how the existence of the crystalline order in solids, ferromagnetism and ferroelectricity, are all the result of symmetry breaking. The consequences of breaking symmetry are that systems show some kind of rigidity (in the case of ferromagnetism this is permanent magnetism), low temperature elementary excitations (in the case of ferromagnetism these are spin waves, also known as magnons), and defects (in the case of ferromagnetism these are domain walls). -

Basic Design and Engineering of Normal-Conducting, Iron-Dominated Electromagnets
Basic design and engineering of normal-conducting, iron-dominated electromagnets Th. Zickler CERN, Geneva, Switzerland Abstract The intention of this course is to provide guidance and tools necessary to carry out an analytical design of a simple accelerator magnet. Basic concepts and magnet types will be explained as well as important aspects which should be considered before starting the actual design phase. The central part of this course is dedicated to describing how to develop a basic magnet design. Subjects like the layout of the magnetic circuit, the excitation coils, and the cooling circuits will be discussed. A short introduction to materials for the yoke and coil construction and a brief summary about cost estimates for magnets will complete this topic. 1 Introduction The scope of these lectures is to give an overview of electromagnetic technology as used in and around particle accelerators considering normal-conducting, iron-dominated electromagnets generally restricted to direct current situations where we assume that the voltages generated by the change of flux and possible resulting eddy currents are negligible. Permanent and superconducting magnet technologies as well as special magnets like kickers and septa are not covered in this paper; they were part of dedicated special lectures. It is clear that it is difficult to give a complete and exhaustive summary of magnet design since there are many different magnet types and designs; in principle the design of a magnet is limited only by the laws of physics and the imagination of the magnet designer. Furthermore, each laboratory and each magnet designer or engineer has his own style of approaching a particular magnet design. -

Characterization and Quantification of Magnetic Remanence in Unexploded Ordnance
CHARACTERIZATION AND QUANTIFICATION OF MAGNETIC REMANENCE IN UNEXPLODED ORDNANCE by Whitney Elizabeth Goodrich A thesis submitted to the Faculty and Board of Trustees of the Colorado School of Mines in partial fulfillment of the requirements for the degree of Master of Science (Geophysics). Golden, Colorado Date _______________ Signed: ____________________ Whitney Elizabeth Goodrich Approved: __________________ Dr. Gary Olhoeft Thesis Advisor Golden, Colorado Date ______________ ___________________________ Dr. Terence K. Young Professor and Head Department of Geophysics ii ABSTRACT Under the Range Rule (1997), the Department of Defense defines unexploded ordnance (UXO) as a piece of ordnance that has been deployed, but has not exploded. This study investigated a subset of UXO including artillery shells and mortars (projectiles), but excluding landmines. UXO contaminates approximately 15 million acres of land in the United States alone. The geophysical tools most frequently used for detection are electromagnetic and magnetic methods. These methods, however, produce a “hit” for any metallic object in the ground, not just UXO. In a given survey, this results in a large number of anomalies, only a small subset of which are actually UXO. Current practice dictates that most anomalies are dug up and identified, and the UXO is exploded in place. The estimated cost to remediate existing UXO, in the United States, with current methods, is tens to hundreds of billions of dollars. Discrimination is the process in which UXO (hazardous) is differentiated from non-UXO (non-hazardous), prior to anomaly excavation. Methods to discriminate the UXO greatly reduce the cost of remediation. Remanent magnetization in UXO is one quantity that must be understood to improve discrimination methodologies in the future. -

Design, Synthesis and Magnetism of Single-Molecule Magnets with Large Anisotropic Barriers
Design, Synthesis and Magnetism of Single-Molecule Magnets with Large Anisotropic Barriers Po-Heng Lin PhD. thesis submitted to the Faculty of Graduate and Postdoctoral Studies In partial fulfillment of the requirements For the PhD. Degree in the Faculty of Science, Department of Chemistry Supervisor: Dr. Muralee Murugesu Department of Chemistry Faculty of Science University of Ottawa © Po-Heng Lin, Ottawa, Canada, 2012 Page | i Abstract This thesis will present the synthesis, characterization and magnetic measurements of III III III lanthanide complexes with varying nuclearities (Ln, Ln2, Ln3 and Ln4). Eu , Gd , Tb , DyIII, HoIII and YbIII have been selected as the metal centers. Eight polydentate Schiff-base ligands have been synthesized with N- and mostly O-based coordination environments which chelate 7-, 8- or 9-coordinate lanthanide ions. The molecular structures were characterized by single crystal X-ray crystallography and the magnetic properties were measured using a SQUID magnetometer. Each chapter consists of crystal structures and magnetic measurements for complexes with the same nuclearity. There are eight DyIII SMMs in this thesis which are discrete molecules that act as magnets below a certain temperature called their blocking temperature. This phenomenon results from an appreciable spin ground state (S) as well as negative uni-axial anisotropy (D), both present in lanthanide ions owing to their f electron shell, generating an effective energy barrier for the reversal of the magnetization (Ueff). The ab initio calculations are also included for the SMMs with high anisotropic energy barriers to understand the mechanisms of slow magnetic relaxation in these systems. Page | ii Acknowledgements and Contributions There are many people who deserve a special mention for their invaluable help with this research project: First and foremost my supervisor Prof. -
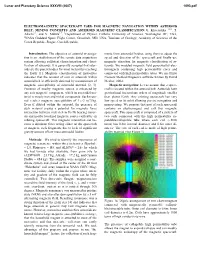
Electromagnetic Spacecraft Used for Magnetic Navigation Within Asteroid Belt, Mining Concepts and Asteroid Magnetic Classification G
Lunar and Planetary Science XXXVIII (2007) 1093.pdf ELECTROMAGNETIC SPACECRAFT USED FOR MAGNETIC NAVIGATION WITHIN ASTEROID BELT, MINING CONCEPTS AND ASTEROID MAGNETIC CLASSIFICATION G. Kletetschka 1,2,3, T. Adachi1,2, and V. Mikula1,2, 1Department of Physics, Catholic University of America, Washington DC, USA, 2NASA Goddard Space Flight Center, Greenbelt, MD, USA, 3Institute of Geology, Academy of Sciences of the Czech Republic, Prague, Czech Republic. Introduction: The objective of asteroid investiga- ments from asteroidal bodies, using them to adjust the tion is an establishment of the remote data acquisition speed and direction of the spacecraft and finally use system allowing sufficient characterization and classi- magnetic attraction for magnetic classification of as- fication of asteroids. It is generally accepted that aster- teroids. We modeled magnetic field generated by elec- oids are the parent bodies for most meteorites reaching tromagnets containing high permeability cores and the Earth [1]. Magnetic classification of meteorites connected with high permeability tubes. We use Finite indicates that the amount of iron in asteroids within Element Method Magnetics software written by David asteroid belt is efficiently detected by measurement of Meeker, 2004. magnetic susceptibility of asteroidal material [2, 3]. Magnetic navigation: Let us assume that a space- Presence of nearby magnetic source is enhanced by craft is located within the asteroid belt. Asteroids have any soft magnetic component, which in asteroidal ma- gravitational interactions orders of magnitude smaller terial is mostly iron and nickel compounds. Such mate- than planet Earth. Any orbiting spacecraft has very rial reaches magnetic susceptibility of 1 e-3 m^3/kg. low speed on its orbit allowing precise navigation and Even if diluted within the asteroid, the presence of maneuvering. -

Hot on the Trail of Residual Magnetism
4 Products and technology A new way of testing current transformers Hot on the trail of residual magnetism Current transformers play an important role in Testing of protection current transformers (CTs) the protection of electrical power systems. They Conventional testing methods apply a signal to one side of the CT and read the resulting output signal on the other side. However, provide the protection relay with a current pro- these methods can be time-consuming and use a lot of equip- portional to the line current so that it can iden- ment. Sometimes they are not even feasible as very high currents tify abnormal conditions and operate according are required, e.g. for on-site testing of current transformers to its settings. The transformation of the current designed for transient behavior (TP, TPX, TPY, TPZ types). As these conventional methods have limitations, OMICRON has developed values from primary to secondary must be ac- a new way of testing CTs. curate during normal operation and especially during system fault conditions (when overcur- Modeling concept rents up to 30-times the nominal current are not This led to the development of the CT Analyzer – a test device using a revolutionary testing concept. The new concept of model- an exception). ing a current transformer allows for a detailed view of its design and physical behavior to be created using parameters measured during the test. It then compares the model with the relevant OMICRON Magazine | Volume 1 Issue 2 2010 Products and technology 5 specification to confirm the accuracy of the CT. The CT Analyzer Different test devices and methods are used to verify the per- is small, lightweight and provides fully automated test plans, formance of current transformers during their development, keeping testing times as short as possible.