A Topological Framework for the Computation of the HOMFLY Polynomial and Its Application to Proteins
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coefficients of Homfly Polynomial and Kauffman Polynomial Are Not Finite Type Invariants
COEFFICIENTS OF HOMFLY POLYNOMIAL AND KAUFFMAN POLYNOMIAL ARE NOT FINITE TYPE INVARIANTS GYO TAEK JIN AND JUNG HOON LEE Abstract. We show that the integer-valued knot invariants appearing as the nontrivial coe±cients of the HOMFLY polynomial, the Kau®man polynomial and the Q-polynomial are not of ¯nite type. 1. Introduction A numerical knot invariant V can be extended to have values on singular knots via the recurrence relation V (K£) = V (K+) ¡ V (K¡) where K£, K+ and K¡ are singular knots which are identical outside a small ball in which they di®er as shown in Figure 1. V is said to be of ¯nite type or a ¯nite type invariant if there is an integer m such that V vanishes for all singular knots with more than m singular double points. If m is the smallest such integer, V is said to be an invariant of order m. q - - - ¡@- @- ¡- K£ K+ K¡ Figure 1 As the following proposition states, every nontrivial coe±cient of the Alexander- Conway polynomial is a ¯nite type invariant [1, 6]. Theorem 1 (Bar-Natan). Let K be a knot and let 2 4 2m rK (z) = 1 + a2(K)z + a4(K)z + ¢ ¢ ¢ + a2m(K)z + ¢ ¢ ¢ be the Alexander-Conway polynomial of K. Then a2m is a ¯nite type invariant of order 2m for any positive integer m. The coe±cients of the Taylor expansion of any quantum polynomial invariant of knots after a suitable change of variable are all ¯nite type invariants [2]. For the Jones polynomial we have Date: October 17, 2000 (561). -

Section 10.2. New Polynomial Invariants
10.2. New Polynomial Invariants 1 Section 10.2. New Polynomial Invariants Note. In this section we mention the Jones polynomial and give a recursion for- mula for the HOMFLY polynomial. We give an example of the computation of a HOMFLY polynomial. Note. Vaughan F. R. Jones introduced a new knot polynomial in: A Polynomial Invariant for Knots via von Neumann Algebra, Bulletin of the American Mathe- matical Society, 12, 103–111 (1985). A copy is available online at the AMS.org website (accessed 4/11/2021). This is now known as the Jones polynomial. It is also a knot invariant and so can be used to potentially distinguish between knots. Shortly after the appearance of Jones’ paper, it was observed that the recursion techniques used to find the Jones polynomial and Conway polynomial can be gen- eralized to a 2-variable polynomial that contains information beyond that of the Jones and Conway polynomials. Note. The 2-variable polynomial was presented in: P. Freyd, D. Yetter, J. Hoste, W.B.R. Lickorish, and A. Ocneanu, A New Polynomial of Knots and Links, Bul- letin of the American Mathematical Society, 12(2): 239–246 (1985). This paper is also available online at the AMS.org website (accessed 4/11/2021). Based on a permutation of the first letters of the names of the authors, this has become knows as the “HOMFLY polynomial.” 10.2. New Polynomial Invariants 2 Definition. The HOMFLY polynomial, PL(`, m), is given by the recursion formula −1 `PL+ (`, m) + ` PL− (`, m) = −mPLS (`, m), and the condition PU (`, m) = 1 for U the unknot. -

Coloring Link Diagrams and Conway-Type Polynomial of Braids
COLORING LINK DIAGRAMS AND CONWAY-TYPE POLYNOMIAL OF BRAIDS MICHAEL BRANDENBURSKY Abstract. In this paper we define and present a simple combinato- rial formula for a 3-variable Laurent polynomial invariant I(a; z; t) of conjugacy classes in Artin braid group Bm. We show that the Laurent polynomial I(a; z; t) satisfies the Conway skein relation and the coef- −k ficients of the 1-variable polynomial t I(a; z; t)ja=1;t=0 are Vassiliev invariants of braids. 1. Introduction. In this work we consider link invariants arising from the Alexander-Conway and HOMFLY-PT polynomials. The HOMFLY-PT polynomial P (L) is an invariant of an oriented link L (see for example [10], [18], [23]). It is a Laurent polynomial in two variables a and z, which satisfies the following skein relation: ( ) ( ) ( ) (1) aP − a−1P = zP : + − 0 The HOMFLY-PT polynomial is normalized( in) the following way. If Or is r−1 a−a−1 the r-component unlink, then P (Or) = z . The Conway polyno- mial r may be defined as r(L) := P (L)ja=1. This polynomial is a renor- malized version of the Alexander polynomial (see for example [9], [17]). All coefficients of r are finite type or Vassiliev invariants. Recently, invariants of conjugacy classes of braids received a considerable attention, since in some cases they define quasi-morphisms on braid groups and induce quasi-morphisms on certain groups of diffeomorphisms of smooth manifolds, see for example [3, 6, 7, 8, 11, 12, 14, 15, 19, 20]. In this paper we present a certain combinatorial construction of a 3-variable Laurent polynomial invariant I(a; z; t) of conjugacy classes in Artin braid group Bm. -

Rotationally Symmetric Rose Links
Rose-Hulman Undergraduate Mathematics Journal Volume 14 Issue 1 Article 3 Rotationally Symmetric Rose Links Amelia Brown Simpson College, [email protected] Follow this and additional works at: https://scholar.rose-hulman.edu/rhumj Recommended Citation Brown, Amelia (2013) "Rotationally Symmetric Rose Links," Rose-Hulman Undergraduate Mathematics Journal: Vol. 14 : Iss. 1 , Article 3. Available at: https://scholar.rose-hulman.edu/rhumj/vol14/iss1/3 Rose- Hulman Undergraduate Mathematics Journal Rotationally Symmetric Rose Links Amelia Browna Volume 14, No. 1, Spring 2013 Sponsored by Rose-Hulman Institute of Technology Department of Mathematics Terre Haute, IN 47803 Email: [email protected] a http://www.rose-hulman.edu/mathjournal Simpson College Rose-Hulman Undergraduate Mathematics Journal Volume 14, No. 1, Spring 2013 Rotationally Symmetric Rose Links Amelia Brown Abstract. This paper is an introduction to rose links and some of their properties. We used a series of invariants to distinguish some rose links that are rotationally symmetric. We were able to distinguish all 3-component rose links and narrow the bounds on possible distinct 4 and 5-component rose links to between 2 and 8, and 2 and 16, respectively. An algorithm for drawing rose links and a table of rose links with up to five components are included. Acknowledgements: The initial cursory observations on rose links were made during the 2011 Dr. Albert H. and Greta A. Bryan Summer Research Program at Simpson College in conjunction with fellow students Michael Comer and Jes Toyne, with Dr. William Schellhorn serving as research advisor. The research presented in this paper, including developing the definitions and related concepts about rose links and applying invariants, was conducted as a part of the author's senior research project at Simpson College, again under the advisement of Dr. -
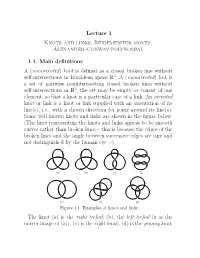
Lecture 1 Knots and Links, Reidemeister Moves, Alexander–Conway Polynomial
Lecture 1 Knots and links, Reidemeister moves, Alexander–Conway polynomial 1.1. Main definitions A(nonoriented) knot is defined as a closed broken line without self-intersections in Euclidean space R3.A(nonoriented) link is a set of pairwise nonintertsecting closed broken lines without self-intersections in R3; the set may be empty or consist of one element, so that a knot is a particular case of a link. An oriented knot or link is a knot or link supplied with an orientation of its line(s), i.e., with a chosen direction for going around its line(s). Some well known knots and links are shown in the figure below. (The lines representing the knots and links appear to be smooth curves rather than broken lines – this is because the edges of the broken lines and the angle between successive edges are tiny and not distinguished by the human eye :-). (a) (b) (c) (d) (e) (f) (g) Figure 1.1. Examples of knots and links The knot (a) is the right trefoil, (b), the left trefoil (it is the mirror image of (a)), (c) is the eight knot), (d) is the granny knot; 1 2 the link (e) is called the Hopf link, (f) is the Whitehead link, and (g) is known as the Borromeo rings. Two knots (or links) K, K0 are called equivalent) if there exists a finite sequence of ∆-moves taking K to K0, a ∆-move being one of the transformations shown in Figure 1.2; note that such a transformation may be performed only if triangle ABC does not intersect any other part of the line(s). -

Knot Polynomials
Knot Polynomials André Schulze & Nasim Rahaman July 24, 2014 1 Why Polynomials? First introduced by James Wadell Alexander II in 1923, knot polynomials have proved themselves by being one of the most efficient ways of classifying knots. In this spirit, one expects two different projections of a knot to have the same knot polynomial; one therefore demands that a good knot polynomial be invariant under the three Reide- meister moves (although this is not always case, as we shall find out). In this report, we present 5 selected knot polynomials: the Bracket, Kauffman X, Jones, Alexander and HOMFLY polynomials. 2 The Bracket Polynomial 2.1 Calculating the Bracket Polynomial The Bracket polynomial makes for a great starting point in constructing knotpoly- nomials. We start with three simple rules, which are then iteratively applied to all crossings in the knot: A direct application of the third rule leads to the following relation for (untangled) unknots: The process of obtaining the Bracket polynomial can be streamlined by evaluating the contribution of a particular sequence of actions in undoing the knot (states) and summing over all such contributions to obtain the net polynomial. 2.2 The Problem with Bracket Polynomials The bracket polynomials can be shown to be invariant under types 2 and 3Reidemeis- ter moves. However by considering type 1 moves, its one major drawback becomes apparent. From: 1 we conclude that that the Bracket polynomial does not remain invariant under type 1 moves. This can be fixed by introducing the writhe of a knot, as we shallsee in the next section. -

New Skein Invariants of Links
NEW SKEIN INVARIANTS OF LINKS LOUIS H. KAUFFMAN AND SOFIA LAMBROPOULOU Abstract. We introduce new skein invariants of links based on a procedure where we first apply the skein relation only to crossings of distinct components, so as to produce collections of unlinked knots. We then evaluate the resulting knots using a given invariant. A skein invariant can be computed on each link solely by the use of skein relations and a set of initial conditions. The new procedure, remarkably, leads to generalizations of the known skein invariants. We make skein invariants of classical links, H[R], K[Q] and D[T ], based on the invariants of knots, R, Q and T , denoting the regular isotopy version of the Homflypt polynomial, the Kauffman polynomial and the Dubrovnik polynomial. We provide skein theoretic proofs of the well- definedness of these invariants. These invariants are also reformulated into summations of the generating invariants (R, Q, T ) on sublinks of a given link L, obtained by partitioning L into collections of sublinks. Contents Introduction1 1. Previous work9 2. Skein theory for the generalized invariants 11 3. Closed combinatorial formulae for the generalized invariants 28 4. Conclusions 38 5. Discussing mathematical directions and applications 38 References 40 Introduction Skein theory was introduced by John Horton Conway in his remarkable paper [16] and in numerous conversations and lectures that Conway gave in the wake of publishing this paper. He not only discovered a remarkable normalized recursive method to compute the classical Alexander polynomial, but he formulated a generalized invariant of knots and links that is called skein theory. -

Polynomial Invariants and Vassiliev Invariants 1 Introduction
ISSN 1464-8997 (on line) 1464-8989 (printed) 89 eometry & opology onographs G T M Volume 4: Invariants of knots and 3-manifolds (Kyoto 2001) Pages 89–101 Polynomial invariants and Vassiliev invariants Myeong-Ju Jeong Chan-Young Park Abstract We give a criterion to detect whether the derivatives of the HOMFLY polynomial at a point is a Vassiliev invariant or not. In partic- (m,n) ular, for a complex number b we show that the derivative PK (b, 0) = ∂m ∂n ∂am ∂xn PK (a, x) (a,x)=(b,0) of the HOMFLY polynomial of a knot K at (b, 0) is a Vassiliev| invariant if and only if b = 1. Also we analyze the ± space Vn of Vassiliev invariants of degree n for n =1, 2, 3, 4, 5 by using the ¯–operation and the ∗ –operation in [5].≤ These two operations are uni- fied to the ˆ –operation. For each Vassiliev invariant v of degree n,v ˆ is a Vassiliev invariant of degree n and the valuev ˆ(K) of a knot≤ K is a polynomial with multi–variables≤ of degree n and we give some questions on polynomial invariants and the Vassiliev≤ invariants. AMS Classification 57M25 Keywords Knots, Vassiliev invariants, double dating tangles, knot poly- nomials 1 Introduction In 1990, V. A. Vassiliev introduced the concept of a finite type invariant of knots, called Vassiliev invariants [13]. There are some analogies between Vassiliev invariants and polynomials. For example, in 1996 D. Bar–Natan showed that when a Vassiliev invariant of degree m is evaluated on a knot diagram having n crossings, the result is approximately bounded by a constant times of nm [2] and S. -

An Introduction to Knot Theory
An Introduction to Knot Theory Matt Skerritt (c9903032) June 27, 2003 1 Introduction A knot, mathematically speaking, is a closed curve sitting in three dimensional space that does not intersect itself. Intuitively if we were to take a piece of string, cord, or the like, tie a knot in it and then glue the loose ends together, we would have a knot. It should be impossible to untangle the knot without cutting the string somewhere. The use of the word `should' here is quite deliberate, however. It is possible, if we have not tangled the string very well, that we will be able to untangle the mess we created and end up with just a circle of string. Of course, this circle of string still ¯ts the de¯nition of a closed curve sitting in three dimensional space and so is still a knot, but it's not very interesting. We call such a knot the trivial knot or the unknot. Knots come in many shapes and sizes from small and simple like the unknot through to large and tangled and messy, and beyond (and everything in between). The biggest questions to a knot theorist are \are these two knots the same or di®erent" or even more importantly \is there an easy way to tell if two knots are the same or di®erent". This is the heart of knot theory. Merely looking at two tangled messes is almost never su±cient to tell them apart, at least not in any interesting cases. Consider the following four images for example. -

A New Symmetry of the Colored Alexander Polynomial
ITEP/TH-04/20 IITP/TH-04/20 MIPT/TH-04/20 A new symmetry of the colored Alexander polynomial V. Mishnyakov a,c,d,∗ A. Sleptsova,b,c,† N. Tselousova,c‡ a Institute for Theoretical and Experimental Physics, Moscow 117218, Russia b Institute for Information Transmission Problems, Moscow 127994, Russia c Moscow Institute of Physics and Technology, Dolgoprudny 141701, Russia d Institute for Theoretical and Mathematical Physics, Moscow State University, Moscow 119991, Russia Abstract We present a new conjectural symmetry of the colored Alexander polynomial, that is the specializa- tion of the quantum slN invariant widely known as the colored HOMFLY-PT polynomial. We provide arguments in support of the existence of the symmetry by studying the loop expansion and the character expansion of the colored HOMFLY-PT polynomial. We study the constraints this symmetry imposes on the group theoretic structure of the loop expansion and provide solutions to those constraints. The symmetry is a powerful tool for research on polynomial knot invariants and in the end we suggest several possible applications of the symmetry. 1 Introduction The colored HOMFLY polynomial is a topological link invariant. It attracts a lot of attention because it is connected to various topics in theoretical and mathematical physics: quantum field theories [5, 6, 7], quantum groups [11, 12, 8], conformal field theories [9], topological strings [10]. Whenever an explicit calculation of a class of HOMFLY invariants is derived it causes advancements in these areas. However, the computations be- come extremely difficult as the representation gets larger. At present moment explicit expressions are available only for several classes of knots and representations, including symmetric and anti-symmetric representations. -

On the Study of Chirality of Knots Through Polynomial Invariants
Treball final de grau GRAU DE MATEMÀTIQUES Facultat de Matemàtiques i Informàtica Universitat de Barcelona KNOT THEORY: On the study of chirality of knots through polynomial invariants Autor: Sergi Justiniano Claramunt Director: Dr. Javier José Gutiérrez Marín Realitzat a: Departament de Matemàtiques i Informàtica Barcelona, January 18, 2019 Contents Abstract ii Introduction iii 1 Mathematical bases 1 1.1 Definition of a knot . .1 1.2 Equivalence of knots . .4 1.3 Knot projections and diagrams . .6 1.4 Reidemeister moves . .8 1.5 Invariants . .9 1.6 Symmetries, properties and generation of knots . 11 1.7 Tangles and Conway notation . 12 2 Jones Polynomial 15 2.1 Introduction . 15 2.2 Rules of bracket polynomial . 16 2.3 Writhe and invariance of Jones polynomial . 18 2.4 Main theorems and applications . 22 3 HOMFLY and Kauffman polynomials on chirality detection 25 3.1 HOMFLY polynomial . 25 3.2 Kauffman polynomial . 28 3.3 Testing chirality . 31 4 Conclusions 33 Bibliography 35 i Abstract In this project we introduce the theory of knots and specialize in the compu- tation of the knot polynomials. After presenting the Jones polynomial, its two two-variable generalizations are also introduced: the Kauffman and HOMFLY polynomial. Then we study the ability of these polynomials on detecting chirality, obtaining a knot not detected chiral by the HOMFLY polynomial, but detected chiral by the Kauffman polynomial. Introduction The main idea of this project is to give a clear and short introduction to the theory of knots and in particular the utility of knot polynomials on detecting chirality of knots. -

The Ways I Know How to Define the Alexander Polynomial
ALL THE WAYS I KNOW HOW TO DEFINE THE ALEXANDER POLYNOMIAL KYLE A. MILLER Abstract. These began as notes for a talk given at the Student 3-manifold seminar, Spring 2019. There seems to be many ways to define the Alexander polynomial, all of which are somehow interrelated, but sometimes there is not an obvious path between any two given definitions. As the title suggests, this is an exploration of all the ways I know how to define this knot invariant. While we will touch on a number of facts and properties, these notes are not meant to be a complete survey of the Alexander polynomial. Contents 1. Introduction2 2. Alexander’s definition2 2.1. The Dehn presentation2 2.2. Abelianization4 2.3. The associated matrix4 3. The Alexander modules6 3.1. Orders7 3.2. Elementary ideals8 3.3. The Fox calculus 10 3.4. Torus knots 13 3.5. The Wirtinger presentation 13 3.6. Generalization to links 15 3.7. Fibered knots 15 3.8. Seifert presentation 16 3.9. Duality 18 4. The Conway potential 18 4.1. Alexander’s three-term relation 20 5. The HOMFLY-PT polynomial 24 6. Kauffman state sum 26 6.1. Another state sum 29 7. The Burau representation 29 8. Vassiliev invariants 29 9. The Alexander quandle 29 9.1. Projective Alexander quandles 33 10. Reidemeister torsion 33 11. Knot Floer homology 33 References 33 Date: May 3, 2019. 1 DRAFT 2020/11/14 18:57:28 2 KYLE A. MILLER 1. Introduction Recall that a link is an embedded closed 1-manifold in S3, and a knot is a 1-component link.