A Knot Polynomial Invariant for Analysis of Topology of RNA Stems and Protein Disulfide Bonds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Symmetry Motivated Link Table
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 15 August 2018 doi:10.20944/preprints201808.0265.v1 Peer-reviewed version available at Symmetry 2018, 10, 604; doi:10.3390/sym10110604 Article A Symmetry Motivated Link Table Shawn Witte1, Michelle Flanner2 and Mariel Vazquez1,2 1 UC Davis Mathematics 2 UC Davis Microbiology and Molecular Genetics * Correspondence: [email protected] Abstract: Proper identification of oriented knots and 2-component links requires a precise link 1 nomenclature. Motivated by questions arising in DNA topology, this study aims to produce a 2 nomenclature unambiguous with respect to link symmetries. For knots, this involves distinguishing 3 a knot type from its mirror image. In the case of 2-component links, there are up to sixteen possible 4 symmetry types for each topology. The study revisits the methods previously used to disambiguate 5 chiral knots and extends them to oriented 2-component links with up to nine crossings. Monte Carlo 6 simulations are used to report on writhe, a geometric indicator of chirality. There are ninety-two 7 prime 2-component links with up to nine crossings. Guided by geometrical data, linking number and 8 the symmetry groups of 2-component links, a canonical link diagram for each link type is proposed. 9 2 2 2 2 2 2 All diagrams but six were unambiguously chosen (815, 95, 934, 935, 939, and 941). We include complete 10 tables for prime knots with up to ten crossings and prime links with up to nine crossings. We also 11 prove a result on the behavior of the writhe under local lattice moves. -
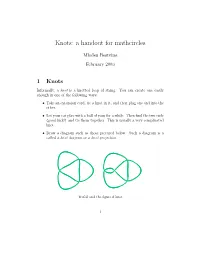
Knots: a Handout for Mathcircles
Knots: a handout for mathcircles Mladen Bestvina February 2003 1 Knots Informally, a knot is a knotted loop of string. You can create one easily enough in one of the following ways: • Take an extension cord, tie a knot in it, and then plug one end into the other. • Let your cat play with a ball of yarn for a while. Then find the two ends (good luck!) and tie them together. This is usually a very complicated knot. • Draw a diagram such as those pictured below. Such a diagram is a called a knot diagram or a knot projection. Trefoil and the figure 8 knot 1 The above two knots are the world's simplest knots. At the end of the handout you can see many more pictures of knots (from Robert Scharein's web site). The same picture contains many links as well. A link consists of several loops of string. Some links are so famous that they have names. For 2 2 3 example, 21 is the Hopf link, 51 is the Whitehead link, and 62 are the Bor- romean rings. They have the feature that individual strings (or components in mathematical parlance) are untangled (or unknotted) but you can't pull the strings apart without cutting. A bit of terminology: A crossing is a place where the knot crosses itself. The first number in knot's \name" is the number of crossings. Can you figure out the meaning of the other number(s)? 2 Reidemeister moves There are many knot diagrams representing the same knot. For example, both diagrams below represent the unknot. -

An Introduction to Knot Theory and the Knot Group
AN INTRODUCTION TO KNOT THEORY AND THE KNOT GROUP LARSEN LINOV Abstract. This paper for the University of Chicago Math REU is an expos- itory introduction to knot theory. In the first section, definitions are given for knots and for fundamental concepts and examples in knot theory, and motivation is given for the second section. The second section applies the fun- damental group from algebraic topology to knots as a means to approach the basic problem of knot theory, and several important examples are given as well as a general method of computation for knot diagrams. This paper assumes knowledge in basic algebraic and general topology as well as group theory. Contents 1. Knots and Links 1 1.1. Examples of Knots 2 1.2. Links 3 1.3. Knot Invariants 4 2. Knot Groups and the Wirtinger Presentation 5 2.1. Preliminary Examples 5 2.2. The Wirtinger Presentation 6 2.3. Knot Groups for Torus Knots 9 Acknowledgements 10 References 10 1. Knots and Links We open with a definition: Definition 1.1. A knot is an embedding of the circle S1 in R3. The intuitive meaning behind a knot can be directly discerned from its name, as can the motivation for the concept. A mathematical knot is just like a knot of string in the real world, except that it has no thickness, is fixed in space, and most importantly forms a closed loop, without any loose ends. For mathematical con- venience, R3 in the definition is often replaced with its one-point compactification S3. Of course, knots in the real world are not fixed in space, and there is no interesting difference between, say, two knots that differ only by a translation. -

Categorified Invariants and the Braid Group
PROCEEDINGS OF THE AMERICAN MATHEMATICAL SOCIETY Volume 143, Number 7, July 2015, Pages 2801–2814 S 0002-9939(2015)12482-3 Article electronically published on February 26, 2015 CATEGORIFIED INVARIANTS AND THE BRAID GROUP JOHN A. BALDWIN AND J. ELISENDA GRIGSBY (Communicated by Daniel Ruberman) Abstract. We investigate two “categorified” braid conjugacy class invariants, one coming from Khovanov homology and the other from Heegaard Floer ho- mology. We prove that each yields a solution to the word problem but not the conjugacy problem in the braid group. In particular, our proof in the Khovanov case is completely combinatorial. 1. Introduction Recall that the n-strand braid group Bn admits the presentation σiσj = σj σi if |i − j|≥2, Bn = σ1,...,σn−1 , σiσj σi = σjσiσj if |i − j| =1 where σi corresponds to a positive half twist between the ith and (i + 1)st strands. Given a word w in the generators σ1,...,σn−1 and their inverses, we will denote by σ(w) the corresponding braid in Bn. Also, we will write σ ∼ σ if σ and σ are conjugate elements of Bn. As with any group described in terms of generators and relations, it is natural to look for combinatorial solutions to the word and conjugacy problems for the braid group: (1) Word problem: Given words w, w as above, is σ(w)=σ(w)? (2) Conjugacy problem: Given words w, w as above, is σ(w) ∼ σ(w)? The fastest known algorithms for solving Problems (1) and (2) exploit the Gar- side structure(s) of the braid group (cf. -

A Computation of Knot Floer Homology of Special (1,1)-Knots
A COMPUTATION OF KNOT FLOER HOMOLOGY OF SPECIAL (1,1)-KNOTS Jiangnan Yu Department of Mathematics Central European University Advisor: Andras´ Stipsicz Proposal prepared by Jiangnan Yu in part fulfillment of the degree requirements for the Master of Science in Mathematics. CEU eTD Collection 1 Acknowledgements I would like to thank Professor Andras´ Stipsicz for his guidance on writing this thesis, and also for his teaching and helping during the master program. From him I have learned a lot knowledge in topol- ogy. I would also like to thank Central European University and the De- partment of Mathematics for accepting me to study in Budapest. Finally I want to thank my teachers and friends, from whom I have learned so much in math. CEU eTD Collection 2 Abstract We will introduce Heegaard decompositions and Heegaard diagrams for three-manifolds and for three-manifolds containing a knot. We define (1,1)-knots and explain the method to obtain the Heegaard diagram for some special (1,1)-knots, and prove that torus knots and 2- bridge knots are (1,1)-knots. We also define the knot Floer chain complex by using the theory of holomorphic disks and their moduli space, and give more explanation on the chain complex of genus-1 Heegaard diagram. Finally, we compute the knot Floer homology groups of the trefoil knot and the (-3,4)-torus knot. 1 Introduction Knot Floer homology is a knot invariant defined by P. Ozsvath´ and Z. Szabo´ in [6], using methods of Heegaard diagrams and moduli theory of holomorphic discs, combined with homology theory. -

Knot Theory and the Alexander Polynomial
Knot Theory and the Alexander Polynomial Reagin Taylor McNeill Submitted to the Department of Mathematics of Smith College in partial fulfillment of the requirements for the degree of Bachelor of Arts with Honors Elizabeth Denne, Faculty Advisor April 15, 2008 i Acknowledgments First and foremost I would like to thank Elizabeth Denne for her guidance through this project. Her endless help and high expectations brought this project to where it stands. I would Like to thank David Cohen for his support thoughout this project and through- out my mathematical career. His humor, skepticism and advice is surely worth the $.25 fee. I would also like to thank my professors, peers, housemates, and friends, particularly Kelsey Hattam and Katy Gerecht, for supporting me throughout the year, and especially for tolerating my temporary insanity during the final weeks of writing. Contents 1 Introduction 1 2 Defining Knots and Links 3 2.1 KnotDiagramsandKnotEquivalence . ... 3 2.2 Links, Orientation, and Connected Sum . ..... 8 3 Seifert Surfaces and Knot Genus 12 3.1 SeifertSurfaces ................................. 12 3.2 Surgery ...................................... 14 3.3 Knot Genus and Factorization . 16 3.4 Linkingnumber.................................. 17 3.5 Homology ..................................... 19 3.6 TheSeifertMatrix ................................ 21 3.7 TheAlexanderPolynomial. 27 4 Resolving Trees 31 4.1 Resolving Trees and the Conway Polynomial . ..... 31 4.2 TheAlexanderPolynomial. 34 5 Algebraic and Topological Tools 36 5.1 FreeGroupsandQuotients . 36 5.2 TheFundamentalGroup. .. .. .. .. .. .. .. .. 40 ii iii 6 Knot Groups 49 6.1 TwoPresentations ................................ 49 6.2 The Fundamental Group of the Knot Complement . 54 7 The Fox Calculus and Alexander Ideals 59 7.1 TheFreeCalculus ............................... -

How Can We Say 2 Knots Are Not the Same?
How can we say 2 knots are not the same? SHRUTHI SRIDHAR What’s a knot? A knot is a smooth embedding of the circle S1 in IR3. A link is a smooth embedding of the disjoint union of more than one circle Intuitively, it’s a string knotted up with ends joined up. We represent it on a plane using curves and ‘crossings’. The unknot A ‘figure-8’ knot A ‘wild’ knot (not a knot for us) Hopf Link Two knots or links are the same if they have an ambient isotopy between them. Representing a knot Knots are represented on the plane with strands and crossings where 2 strands cross. We call this picture a knot diagram. Knots can have more than one representation. Reidemeister moves Operations on knot diagrams that don’t change the knot or link Reidemeister moves Theorem: (Reidemeister 1926) Two knot diagrams are of the same knot if and only if one can be obtained from the other through a series of Reidemeister moves. Crossing Number The minimum number of crossings required to represent a knot or link is called its crossing number. Knots arranged by crossing number: Knot Invariants A knot/link invariant is a property of a knot/link that is independent of representation. Trivial Examples: • Crossing number • Knot Representations / ~ where 2 representations are equivalent via Reidemester moves Tricolorability We say a knot is tricolorable if the strands in any projection can be colored with 3 colors such that every crossing has 1 or 3 colors and or the coloring uses more than one color. -

UW Math Circle May 26Th, 2016
UW Math Circle May 26th, 2016 We think of a knot (or link) as a piece of string (or multiple pieces of string) that we can stretch and move around in space{ we just aren't allowed to cut the string. We draw a knot on piece of paper by arranging it so that there are two strands at every crossing and by indicating which strand is above the other. We say two knots are equivalent if we can arrange them so that they are the same. 1. Which of these knots do you think are equivalent? Some of these have names: the first is the unkot, the next is the trefoil, and the third is the figure eight knot. 2. Find a way to determine all the knots that have just one crossing when you draw them in the plane. Show that all of them are equivalent to an unknotted circle. The Reidemeister moves are operations we can do on a diagram of a knot to get a diagram of an equivalent knot. In fact, you can get every equivalent digram by doing Reidemeister moves, and by moving the strands around without changing the crossings. Here are the Reidemeister moves (we also include the mirror images of these moves). We want to have a way to distinguish knots and links from one another, so we want to extract some information from a digram that doesn't change when we do Reidemeister moves. Say that a crossing is postively oriented if it you can rotate it so it looks like the left hand picture, and negatively oriented if you can rotate it so it looks like the right hand picture (this depends on the orientation you give the knot/link!) For a link with two components, define the linking number to be the absolute value of the number of positively oriented crossings between the two different components of the link minus the number of negatively oriented crossings between the two different components, #positive crossings − #negative crossings divided by two. -

An Introduction to the Theory of Knots
An Introduction to the Theory of Knots Giovanni De Santi December 11, 2002 Figure 1: Escher’s Knots, 1965 1 1 Knot Theory Knot theory is an appealing subject because the objects studied are familiar in everyday physical space. Although the subject matter of knot theory is familiar to everyone and its problems are easily stated, arising not only in many branches of mathematics but also in such diverse fields as biology, chemistry, and physics, it is often unclear how to apply mathematical techniques even to the most basic problems. We proceed to present these mathematical techniques. 1.1 Knots The intuitive notion of a knot is that of a knotted loop of rope. This notion leads naturally to the definition of a knot as a continuous simple closed curve in R3. Such a curve consists of a continuous function f : [0, 1] → R3 with f(0) = f(1) and with f(x) = f(y) implying one of three possibilities: 1. x = y 2. x = 0 and y = 1 3. x = 1 and y = 0 Unfortunately this definition admits pathological or so called wild knots into our studies. The remedies are either to introduce the concept of differentiability or to use polygonal curves instead of differentiable ones in the definition. The simplest definitions in knot theory are based on the latter approach. Definition 1.1 (knot) A knot is a simple closed polygonal curve in R3. The ordered set (p1, p2, . , pn) defines a knot; the knot being the union of the line segments [p1, p2], [p2, p3],..., [pn−1, pn], and [pn, p1]. -

Coloring Link Diagrams and Conway-Type Polynomial of Braids
COLORING LINK DIAGRAMS AND CONWAY-TYPE POLYNOMIAL OF BRAIDS MICHAEL BRANDENBURSKY Abstract. In this paper we define and present a simple combinato- rial formula for a 3-variable Laurent polynomial invariant I(a; z; t) of conjugacy classes in Artin braid group Bm. We show that the Laurent polynomial I(a; z; t) satisfies the Conway skein relation and the coef- −k ficients of the 1-variable polynomial t I(a; z; t)ja=1;t=0 are Vassiliev invariants of braids. 1. Introduction. In this work we consider link invariants arising from the Alexander-Conway and HOMFLY-PT polynomials. The HOMFLY-PT polynomial P (L) is an invariant of an oriented link L (see for example [10], [18], [23]). It is a Laurent polynomial in two variables a and z, which satisfies the following skein relation: ( ) ( ) ( ) (1) aP − a−1P = zP : + − 0 The HOMFLY-PT polynomial is normalized( in) the following way. If Or is r−1 a−a−1 the r-component unlink, then P (Or) = z . The Conway polyno- mial r may be defined as r(L) := P (L)ja=1. This polynomial is a renor- malized version of the Alexander polynomial (see for example [9], [17]). All coefficients of r are finite type or Vassiliev invariants. Recently, invariants of conjugacy classes of braids received a considerable attention, since in some cases they define quasi-morphisms on braid groups and induce quasi-morphisms on certain groups of diffeomorphisms of smooth manifolds, see for example [3, 6, 7, 8, 11, 12, 14, 15, 19, 20]. In this paper we present a certain combinatorial construction of a 3-variable Laurent polynomial invariant I(a; z; t) of conjugacy classes in Artin braid group Bm. -

The Kauffman Bracket and Genus of Alternating Links
California State University, San Bernardino CSUSB ScholarWorks Electronic Theses, Projects, and Dissertations Office of aduateGr Studies 6-2016 The Kauffman Bracket and Genus of Alternating Links Bryan M. Nguyen Follow this and additional works at: https://scholarworks.lib.csusb.edu/etd Part of the Other Mathematics Commons Recommended Citation Nguyen, Bryan M., "The Kauffman Bracket and Genus of Alternating Links" (2016). Electronic Theses, Projects, and Dissertations. 360. https://scholarworks.lib.csusb.edu/etd/360 This Thesis is brought to you for free and open access by the Office of aduateGr Studies at CSUSB ScholarWorks. It has been accepted for inclusion in Electronic Theses, Projects, and Dissertations by an authorized administrator of CSUSB ScholarWorks. For more information, please contact [email protected]. The Kauffman Bracket and Genus of Alternating Links A Thesis Presented to the Faculty of California State University, San Bernardino In Partial Fulfillment of the Requirements for the Degree Master of Arts in Mathematics by Bryan Minh Nhut Nguyen June 2016 The Kauffman Bracket and Genus of Alternating Links A Thesis Presented to the Faculty of California State University, San Bernardino by Bryan Minh Nhut Nguyen June 2016 Approved by: Dr. Rolland Trapp, Committee Chair Date Dr. Gary Griffing, Committee Member Dr. Jeremy Aikin, Committee Member Dr. Charles Stanton, Chair, Dr. Corey Dunn Department of Mathematics Graduate Coordinator, Department of Mathematics iii Abstract Giving a knot, there are three rules to help us finding the Kauffman bracket polynomial. Choosing knot's orientation, then applying the Seifert algorithm to find the Euler characteristic and genus of its surface. Finally finding the relationship of the Kauffman bracket polynomial and the genus of the alternating links is the main goal of this paper. -

Knot Topology in Quantum Spin System
Knot Topology in Quantum Spin System X. M. Yang, L. Jin,∗ and Z. Songy School of Physics, Nankai University, Tianjin 300071, China Knot theory provides a powerful tool for the understanding of topological matters in biology, chemistry, and physics. Here knot theory is introduced to describe topological phases in the quantum spin system. Exactly solvable models with long-range interactions are investigated, and Majorana modes of the quantum spin system are mapped into different knots and links. The topological properties of ground states of the spin system are visualized and characterized using crossing and linking numbers, which capture the geometric topologies of knots and links. The interactivity of energy bands is highlighted. In gapped phases, eigenstate curves are tangled and braided around each other forming links. In gapless phases, the tangled eigenstate curves may form knots. Our findings provide an alternative understanding of the phases in the quantum spin system, and provide insights into one-dimension topological phases of matter. Introduction.|Knots are categorized in terms of ge- topological systems. The ground states of the quantum ometric topology, and describe the topological proper- spin system are mapped into knots and links; the topo- ties of one-dimensional (1D) closed curves in a three- logical invariants constructed from eigenstates are then dimensional space [1, 2]. A collection of knots without directly visualized; and topological features are revealed an intersection forms a link. The significance of knots in from the geometric topologies of knots and links. In con- science is elusive; however, knot theory can be used to trast to the conventional description of band topologies, characterize the topologies of the DNA structure [3] and such as the Zak phase and Chern number that can be synthesized molecular structure [4] in biology and chem- extracted from a single band, this approach highlights istry.