Killing of Organisms Responsible for Wound Infections Using a Light-Activated Antimicrobial Agent
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Identification of Olefins As Thiocyanates
THE IDENTIFICATION OF OLEFI NS AS THIOCYANATES 1 .. .SEP 2"/ i 938 THE IDENTIFICATION OF OLEFINS AS THIOCYANAT ES/ ( ' By George A. Dysinger I\ Bachelor of Science Oklahoma Agricultural and Mechanical College Stillwater, Oklahoma 1937 Submitted to the Department of Chemistry Oklahoma Agricultural and Mechanical College In partial fulfillme.nt of the requirements for the Degree of MASTER OF SCIENCE 1938 . ... .. '.'' .. ~ . ..... .. • • • • • • • • J • : ... ·:· .· ~- . .. ,. r f • • • - • • • • •• J • •• ; • • • ii !)fp 27 1938 APPROVED: HeadOttinm~- of : e Department of Onemistry ~~~e~ 108550 iii ACKNOWLEDGEMENT The author wishes to expres.s his sincere appreciation to Dr. o. c. Dermer under whose direction and with whose help this work has been done. He also wishes to acknowledge the assistance rendered by Everett L. Ada.ms in supplying apparatus and chemicals. iv TABLE OF CONTENTS Page Introduotion • . .. • . • • • 1 Historical Review. • . • • . • . 2 Experimental . • • • • . • . • . • • 6 Discussion of Results . .. ao Summary • • • • . • • • 22 Bibliography • • • • • • • • • • • • • • • • • • 23 Autobiography • • • • • . • • • • • • • • • . • 24 1 INTRODUCTION At present, the identification of low boiling unsatu rated hydrocarbons which yield only liquid addition products with the halogens and hydrogen halides is often a matter of considerable difficulty. This f'aot is sufficient reason for the study here described. In some cases, espeoially among terpen.es, the compounds formed by adding NOCl, 1203 , or »2o4 at one or more of the double bonds have been used as derivatives but these are i nconvenient to make,. of un certain composition, and decidedly unstable! This work con- '\, sists of (l) a'ttempts to find a convenient samll scale meth- od for adding (S0N)2 to olef1ns, (2) the determinations of the melting points of the derivatives, and (3) quantitatiye analysis of derivatives to prove their structure and purity. -

Infection at the Wildlife-Livestock-Human Interface: Three Systems
Infection at the Wildlife- livestock-human interface: three systems Thesis submitted in accordance with the requirements of the University of Liverpool for the degree of Doctor in Philosophy by Elsa Sandoval Barron 12/4/2017 Abstract Zoonoses involve interactions between at least three species: the pathogen and two hosts, one of which is human and the other a non-human (vertebrate) animal. More than 60% of human infectious diseases are zoonotic, and many have a wildlife host. Urbanisation and human population growth have increased the demand for food and land resources, which have increased interaction between humans, domestic animals and wildlife and thus the potential for cross-species transmission of infections. Most studies of such systems take place in tropical and developing countries where population change and biodiversity makes the emergence of high profile infections (eg Ebola and SARS) more likely. This study, however, focuses on four well known infections within the UK: bovine tuberculosis, water-borne cryptosporidiosis and giardiasis, and campylobacteriosis. The aim of this study was to investigate, using four infectious diseases of economic and public health importance in the UK as study systems, the role of wildlife in the epidemiology of multihost, zoonotic infections. Bovine tuberculosis (bTB) is an important zoonosis in many parts of the world, but human infection is rare in the UK owing to a policy of ‘test and cull’ in cattle and pasteurisation of milk. However, there has been an epidemic of bTB in British cattle in recent decades, the control of which is complicated by infection in badgers (Meles meles) and controversy over the control of wildlife infection. -

MYCOBACTERIA.Pdf
MYCOBACTERIA Mycobacteria are widespread organisms, typically living in and food sources. Tuberculosis and the leprosy organisms are obligate parasites and are not found as free-living members of the genus. Mycobacteria are aerobic and nonmotile bacteria (except for the species Mycobacterium marinum, which has been shown to be motile within macrophages) that are characteristically acid fast. Mycobacteria have an outer membrane. They do not have capsules, and most do not form endospores. The distinguishing characteristic of all Mycobacterium species is that the cell wall is thicker than in many other bacteria, which is hydrophobic, waxy, and rich in mycolic acids/mycolates. The cell wall consists of the hydrophobic mycolate layer and a peptidoglycan layer held together by a polysaccharide, arabinogalactan. The cell wall makes a substantial contribution to the hardiness of this genus. The biosynthetic pathways of cell wall components are potential targets for new drugs for tuberculosis. Fig. 1 Mycobacterial cell wall: 1-outer lipids, 2-mycolic acid, 3-polysaccharides (arabinogalactan), 4-peptidoglycan, 5-plasma membrane, 6-lipoarabinomannan (LAM), 7- phosphatidylinositol mannoside, 8-cell wall skeleton. NONTUBERCULOUS MYCOBACTERIA – RUNYON CLASSIFICATION Runyon classification is a system of identifying mycobacteria on the basis of pigmentation and growth condition of the organisms. The Runyon classification of nontuberculous mycobacteria based on the rate of growth, production of yellow pigment and whether this pigment was produced in the dark or only after exposure to light. It was introduced by Ernest Runyon in 1959 (Fig. 111). On these bases, the nontuberculous mycobacteria are divided into four groups: Photochromogens (Group I) - produce nonpigmented colonies when grown in the dark and pigmented colonies only after exposure to light and reincubation (1M. -

Streptococcus Pneumoniae Capsular Polysaccharide Is Linked to Peptidoglycan Via a Direct Glycosidic Bond to Β-D-N-Acetylglucosamine
Streptococcus pneumoniae capsular polysaccharide is linked to peptidoglycan via a direct glycosidic bond to β-D-N-acetylglucosamine Thomas R. Larsona and Janet Yothera,1 aDepartment of Microbiology, University of Alabama at Birmingham, Birmingham, AL 35294-2170 Edited by Emil C. Gotschlich, The Rockefeller University, New York, NY, and approved April 14, 2017 (received for review December 20, 2016) For many bacteria, including those important in pathogenesis, (Und-P). In S. pneumoniae serotype 2 CPS, Glcp-1-P is trans- expression of a surface-localized capsular polysaccharide (CPS) can ferred from UDP-Glcp (11), and this is followed by addition of be critical for survival in host environments. In Gram-positive the remaining sugars (12, 13) to form the complete repeat unit bacteria, CPS linkage is to either the cytoplasmic membrane or the (Fig. 1). Und-P-P-oligosaccharide repeat units are translocated cell wall. Despite the frequent occurrence and essentiality of these to the outer face of the cytoplasmic membrane by Wzx and po- polymers, the exact nature of the cell wall linkage has not been lymerized into high molecular weight (MW) polysaccharide by described in any bacterial species. Using the Streptococcus pneu- Wzy. Growth occurs at the reducing end, with single or multiple moniae serotype 2 CPS, which is synthesized by the widespread repeat units being transferred en bloc from Und-P-P to an ac- Wzy mechanism, we found that linkage occurs via the reducing ceptor Und-P-P-oligosaccharide repeat unit. Hydrolysis of the β N- end glucose of CPS and the -D- acetylglucosamine (GlcNAc) res- donor Und-P-P that remains after transfer yields Und-P, which is idues of peptidoglycan (PG). -

Infection Control
infection control JANICE CARR The above photo depicts an E.coli (ATCC 11775) biofilm grown on PC (polycarbonate) coupons using a CDC biofilm reactor. Microorganisms often colonize, and adhere strongly to living and non-living surfaces forming biofilms, and at times, demonstrate an increased resistance to antimicrobials. Biofilms on indwelling medical devices pose a serious threat to public health. BIOFILMS:BIOFILMS: Friend or Foe? By NICOLE KENNY, B.Sc, Assoc.Chem., Director of Professional & Technical Services, Virox Technologies Inc 38 Sanitation Canada - SEPTEMBER / OCTOBER 2006 JANICE CARR Scanning electron micrograph of a Staphylococcus biofilm on the inner surface of a needleless connector. A distinguishing characteristic of biofilms is the presence of extracellular polymeric substances, primarily polysaccharides, surrounding and encasing the cells. Here, there polysaccharides have been visualized by scanning electron microscopy. Picture yourself a con- “Why didn’t I listen to my mother and take Biofilms can be dangerous or benefi- testant on Jeopardy. Alex more science courses?” But in that split cial depending on where they are found Tribec has just asked you second you also remember a documen- and of which organisms they are com- to choose the category. tary you watched on CNN about whirl- prised. In industry, biofilms are responsi- You’re lagging behind the pool tubs and you know the answer. ble for billions of dollars in lost produc- leader by $400. All but “Alex, what are BIOFILMS?” tivity due to equipment damage, notori- one of the $500 questions ously famous for causing pipes to plug or Phave been taken, and the last category has THE ISSUE corrode. -

Functional Anatomy of Prokaryotes and Eukaryotes
FUNCTIONAL ANATOMY OF PROKARYOTES AND EUKARYOTES BY DR JAWAD NAZIR ASSISTANT PROFESSOR DEPARTMENT OF MICROBIOLOGY UNIVERSITY OF VETERINARY AND ANIMAL SCIENCES, LAHORE Prokaryotes vs Eukaryotes Prokaryote comes from the Greek words for pre-nucleus Eukaryote comes from the Greek words for true nucleus. Functional anatomy of prokaryotes Prokaryotes vs Eukaryotes Prokaryotes Eukaryotes One circular chromosome, not in Paired chromosomes, in nuclear a membrane membrane No histones Histones No organelles Organelles Peptidoglycan cell walls Polysaccharide cell walls Binary fission Mitotic spindle Functional anatomy of prokaryotes Size and shape Average size: 0.2 -1.0 µm 2 - 8 µm Basic shapes: Functional anatomy of prokaryotes Size and shape Pairs: diplococci, diplobacilli Clusters: staphylococci Chains: streptococci, streptobacilli Functional anatomy of prokaryotes Size and shape Functional anatomy of prokaryotes Size and shape Functional anatomy of prokaryotes Size and shape Unusual shapes Star-shaped Stella Square Haloarcula Most bacteria are monomorphic A few are pleomorphic Genus: Stella Genus: Haloarcula Functional anatomy of prokaryotes Bacterial cell structure Structures external to cell wall Cell wall itself Structures internal to cell wall Functional anatomy of prokaryotes Glycocalyx Outside cell wall Usually sticky A capsule is neatly organized A slime layer is unorganized & loose Extracellular polysaccharide allows cell to attach Capsules prevent phagocytosis Association with diseases B. anthracis S. pneumoniae Functional anatomy of prokaryotes Flagella Outside cell wall Filament made of chains of flagellin Attached to a protein hook Anchored to the wall and membrane by the basal body Functional anatomy of prokaryotes Flagella Arrangement Functional anatomy of prokaryotes Bacterial motility Rotate flagella to run or tumble Move toward or away from stimuli (taxis) Flagella proteins are H antigens (e.g., E. -

Cell Structure and Function in the Bacteria and Archaea
4 Chapter Preview and Key Concepts 4.1 1.1 DiversityThe Beginnings among theof Microbiology Bacteria and Archaea 1.1. •The BacteriaThe are discovery classified of microorganismsinto several Cell Structure wasmajor dependent phyla. on observations made with 2. theThe microscope Archaea are currently classified into two 2. •major phyla.The emergence of experimental 4.2 Cellscience Shapes provided and Arrangements a means to test long held and Function beliefs and resolve controversies 3. Many bacterial cells have a rod, spherical, or 3. MicroInquiryspiral shape and1: Experimentation are organized into and a specific Scientificellular c arrangement. Inquiry in the Bacteria 4.31.2 AnMicroorganisms Overview to Bacterialand Disease and Transmission Archaeal 4.Cell • StructureEarly epidemiology studies suggested how diseases could be spread and 4. Bacterial and archaeal cells are organized at be controlled the cellular and molecular levels. 5. • Resistance to a disease can come and Archaea 4.4 External Cell Structures from exposure to and recovery from a mild 5.form Pili allowof (or cells a very to attach similar) to surfacesdisease or other cells. 1.3 The Classical Golden Age of Microbiology 6. Flagella provide motility. Our planet has always been in the “Age of Bacteria,” ever since the first 6. (1854-1914) 7. A glycocalyx protects against desiccation, fossils—bacteria of course—were entombed in rocks more than 3 billion 7. • The germ theory was based on the attaches cells to surfaces, and helps observations that different microorganisms years ago. On any possible, reasonable criterion, bacteria are—and always pathogens evade the immune system. have been—the dominant forms of life on Earth. -

Chemical Probes to Visualize Bacterial Cell Structure and Physiology
molecules Review From Differential Stains to Next Generation Physiology: Chemical Probes to Visualize Bacterial Cell Structure and Physiology Jonathan Hira 1, Md. Jalal Uddin 1 , Marius M. Haugland 2 and Christian S. Lentz 1,* 1 Research Group for Host-Microbe Interactions, Department of Medical Biology and Centre for New Antibacterial Strategies (CANS), UiT—The Arctic University of Norway, 9019 Tromsø, Norway; [email protected] (J.H.); [email protected] (M.J.U.) 2 Department of Chemistry and Centre for New Antibacterial Strategies (CANS), UiT—The Arctic University of Norway, 9019 Tromsø, Norway; [email protected] * Correspondence: [email protected] Academic Editor: Steven Verhelst Received: 30 September 2020; Accepted: 23 October 2020; Published: 26 October 2020 Abstract: Chemical probes have been instrumental in microbiology since its birth as a discipline in the 19th century when chemical dyes were used to visualize structural features of bacterial cells for the first time. In this review article we will illustrate the evolving design of chemical probes in modern chemical biology and their diverse applications in bacterial imaging and phenotypic analysis. We will introduce and discuss a variety of different probe types including fluorogenic substrates and activity-based probes that visualize metabolic and specific enzyme activities, metabolic labeling strategies to visualize structural features of bacterial cells, antibiotic-based probes as well as fluorescent conjugates to probe biomolecular uptake pathways. Keywords: activity-based probe; antibiotic conjugate; bacterial imaging; bacterial uptake; fluorogenic substrate; metabolic labeling; phenotypic heterogeneity 1. Introduction—From 19th Century Microbiology to Modern Day Chemical Biology If chemical biology can be defined as the ‘interrogation of biological systems with chemical approaches’ [1], we must acknowledge some of the first microbiologists as chemical biologists. -
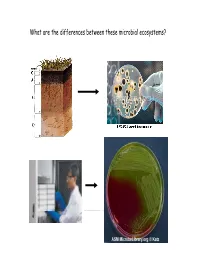
What Are the Differences Between These Microbial Ecosystems? Wwwwe Now Know That, Like Other Org Gm,Anisms, Bacteria Exhibit Social Behaviors
What are the differences between these microbial ecosystems? WWwwe now know that, like other org gm,anisms, bacteria exhibit social behaviors. Bacterial cell escaping for a rare moment of peace and quiet contemplation Sociomicrobiology I. Cell signaling A. Definition/description B. Intraspecific (within spp.): Myxococcus C. Interspecific (between spp.): Pseudomonas aureofaciens II. Biofilms A. Biofilm formation B. Planktonic cells vs. biofilm cells C. General characteristics, structures D. Biofilms as social entities Small diffusible molecules mediate bacterial communication O O N H AHL O O O N H O http://www.ted.com/index.php/talks/bonnie_bassler_on_how_bacteria_communicate.html Why cell-cell signaling in bacteria? Often, single cells in a population might benefit from knowing how many cells are present… “A multitude of bacteria are stronger than a few, thus by union are able overcome obstacles too great for few.” -- Dr. Erwin F. Smith, 1905 (Father of Plant Bacteriology) Pseudomonas aeruginosa in lungs Xylella fastidiosa in xylem Why cell-cell signaling in bacteria? Cell-cell signaling enables bacteria to coordinate behavior to respond quickly to environmental stimuli… such as: -presence of suitable host -change in nutrient availability -defense/competition against other microorganisms -many others! CmmiCommunica tion among btbacteri i:a: The example of Myxococcus xanthus, the Wolf Pack feeder of bacteria http://cmgm.stanford.edu/devbio/kaiserlab/about_myxo/about_myxococcus.html Cooperation among cells in a population: myxobacteria. The myxobacteria are Gram-negative, ubiquitous, soil-dwelling bacteria that are capable of multicellular, social behaviour. In the presence of nutrients, “swarms” of myxobacteria feed cooperatively by sharing extracellular digestive enzymes, and can prey on other bacteria. -

Extraction of Cyanides from Waste Solutions of Cyanidation of .Lotation
Chemistry for Sustainable Development 12 (2004) 431436 431 Extraction of Cyanides from Waste Solutions of Cyanidation of lotation Concentrates from Kholbinskoye Gold Deposit A. A. KOCHANOV1, A. A. RYAZANTSEV1, A. A. BATOEVA2, D. B. ZHALSANOVA2, A. M. BADALYAN3 and O. V. POLYAKOV3 1Siberian Transport University, Ul. D. Kovalchuk 191, Novosibirsk 630049 (Russia) E-mail: [email protected] 2Baikal Institute of Nature Management, Siberian Branch of the Russian Academy of Sciences, Ul. Sakhyanovoy 6, Ulan Ude 670047 (Russia) 3A. V. Nikolaev Institute of Inorganic Chemistry, Siberian Branch of the Russian Academy of Sciences, Pr. Akademika Lavrentyeva 3, Novosibirsk 630090 (Russia) (Received April 21, 2003; in revised form January 6, 2004) Abstract Processes that occur during extraction of cyanides from cyanidation solutions using centrifugal bubbling apparatuses (CBA) as reactors have been studied. In the eddy chamber of CBA (ðÍ < 3), one can observe virtually complete removal of HCN from solution and precipitation of heavy metals in the form of insoluble x - 1 compounds. The electronic absorption spectra of solutions treated in CBA suggest that destruction of [Cu(CN)x] , + 2+ 2 oxidation of Cu to Cu by air oxygen, and oxidation of thiocyanates in the presence of SO23, forming 2 HCN and SO4 , are also accompanied by the emergence of stable intermediate products (SCN)2 and (SCN)x of oxidation in solution. INTRODUCTION formed after acidification of the waste process- ing solutions of cyanidation to ðÍ 62.5 fol- Cyanides are widely used in extraction of lowed by absorption of HCN by alkaline solu- precious metals, mainly fine gold and silver, tions. -

United States Patent Office Patented Feb
3,644,463 United States Patent Office Patented Feb. 22, 1972 2 agent that is introduced. Thus, for example, good yields 3,644,463 of 1,2-vinylene-bisthiocyanate are obtained by adding PRODUCTION OF ALEPHATIC 1,2-BISTHIOCYANATES acetylene gas, while the addition of ethylene results in the Richard Parke Welcher, Old Greenwich, Conn., assignor production of 1,2-dithiocyanoethane. The corresponding to American Cyanamid Company, Stamford, Conn. monoalkyl-substituted vinylene bisthiocyanates are pro No Drawing. Filed May 15, 1968, Ser. No. 729,375 duced when monoalkyl acetylene containing an alkyl radi Int, C. C07c 161/02 cal of from 1 to 16 carbn atoms are used, such as methyl U.S. C. 260-454 5 Claims acetylene, heptyne, octyne, octadecyne, and the like. Monoalkyl-dithiocyanoethanes are produced in similar O manner when monoalkylethylenes containing alkyl radi cals of 1-16 carbon atoms are used; typical of these are ABSTRACT OF THE DISCLOSURE propylene, isobutylene, octylethylene, hexadecyl-ethylene Aliphatic 1,2-bisthiocyanates are produced by first pre and the like. Dialkyl ethylenes may likewise be used, the paring a solution of thiocyanogen in a water-insoluble preferred reagents being those wherein the two alkyl sub liquid organic solvent such as toluene having an aqueous stituents taken together have a total of from 2 to 16 car solution of an inorganic halide admixed therewith, draw bon atoms. Aryl-substituted olefins such as styrene may ing off the aqueous phase, adding an alpha-olefin or acetyl also be used. The principles of the invention can also ene and reacting at a temperature below about 20° C. -

Emerging Frontiers in Microbiome Engineering.Pdf
Trends in Immunology Review Emerging Frontiers in Microbiome Engineering Marı´a Eugenia Inda,1,2,5 Esther Broset,3,4,5 Timothy K. Lu,1,2,* and Cesar de la Fuente-Nunez3,* The gut microbiome has a significant impact on health and disease and can actively contribute to Highlights obesity, diabetes, inflammatory bowel disease, cardiovascular disease, and neurological disor- Themicrobiomeplaysafunda- ders. We do not yet have the necessary tools to fine-tune the microbial communities that consti- mental role in our health and tute the microbiome, though such tools could unlock extensive benefits to human health. Here, disease. we provide an overview of the current state of technological tools that may be used for micro- biome engineering. These tools can enable investigators to define the parameters of a healthy Engineering the microbiome might enable studying the contribution of microbiome and to determine how gut bacteria may contribute to the etiology of a variety of individual microbes and generating diseases. These tools may also allow us to explore the exciting prospect of developing targeted potential therapies against meta- therapies and personalized treatments for microbiome-linked diseases. bolic (e.g., phenylketonuria and chronic kidney disease), inflamma- tory, and immunological diseases, Modulating the Microbiome: An Emerging Paradigm for Understanding and Treating among others. Human Diseases Current methods for probing the The human body is home to at least as many microbial cells as human cells [1]. However, the most microbiome include fecal micro- salient characteristic of the interaction between microbes and the human body is not the number biota transplantation and the use of of cells involved, but their inextricable link with each other.