Lessons Learned from Failed Island Rodent Eradications Redone Successfully:Implications for the Second Rat Eradication Attempt on Wake Atoll
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

House of Assembly Wednesday 18 November 2020
PARLIAMENT OF TASMANIA HOUSE OF ASSEMBLY REPORT OF DEBATES Wednesday 18 November 2020 REVISED EDITION Wednesday 18 November 2020 The Speaker, Ms Hickey, took the Chair at 10 a.m., acknowledged the Traditional People and read Prayers. QUESTIONS Budget 2020-21 - Jobs and Unemployment Figures Ms WHITE question to PREMIER, Mr GUTWEIN [10.02 a.m.] Your Government does not have a plan for jobs. You only have a plan for unemployment. Yesterday the ABS released another bad set of job numbers for Tasmania. Tasmania's recovery continues to go backwards and we are the only state still shedding jobs. Job losses in Tasmania are now at 50 per cent above the national average; 2300 payroll jobs have been lost in the past month, and 900 of those jobs were in construction which makes a mockery of your pledge to build Tasmania out of recession. These are the figures that you are refusing to acknowledge, just as you are ignoring your own Budget forecast for job losses and unemployment above 8 per cent. When are you going to admit that your approach to creating jobs is not working? ANSWER Madam Speaker, I thank the Leader of the Opposition for that question and her interest in this matter. We just delivered a Budget with $5 billion worth of infrastructure in it. A very sensible multiplier when applied to that $5 billion worth of infrastructure would indicate that that Budget will support 25 000 jobs. We will be building roads and bridges. We will be building schools. We will be investing in hospitals and, importantly, we will be building houses. -

FRDC Final Report Design Standard
Understanding broad scale impacts of salmonid farming on rocky reef communities [Valentine, J.P., Jensen, M., Ross, D.J., Riley, S., Ibbott, S.] [July 2016] FRDC Project No 2014/042 Page 1 of 53 © 2016 Fisheries Research and Development Corporation. All rights reserved. ISBN [978-0-646-96251-1] Understanding broad scale impacts of salmonid farming on rocky reef communities FRDC Project No 2014/042 2016 Ownership of Intellectual property rights Unless otherwise noted, copyright (and any other intellectual property rights, if any) in this publication is owned by the Fisheries Research and Development Corporation. This publication (and any information sourced from it) should be attributed to [Valentine, J.P., Jensen, M., Ross, D.J., Riley, S., Ibbott, S., Organisation, 2016, Understanding broad scale impacts of salmonid farming on rocky reef communities, Hobart, September, 2016.] Creative Commons licence All material in this publication is licensed under a Creative Commons Attribution 3.0 Australia Licence, save for content supplied by third parties, logos and the Commonwealth Coat of Arms. Creative Commons Attribution 3.0 Australia Licence is a standard form licence agreement that allows you to copy, distribute, transmit and adapt this publication provided you attribute the work. A summary of the licence terms is available from creativecommons.org/licenses/by/3.0/au/deed.en. The full licence terms are available from creativecommons.org/licenses/by/3.0/au/legalcode. Inquiries regarding the licence and any use of this document should be sent to: [email protected] Disclaimer The authors do not warrant that the information in this document is free from errors or omissions. -

Maria Island MARINE RESERVE
VISITING RESERVES Maria Island MARINE RESERVE Maria Island Marine Reserve protects spectacular underwater seascapes and the most diverse range of marine life in the state, offering excellent snorkelling and diving opportunities. The marine reserve extends along the north and west coasts of the island from low water mark to 20 m water depth. In the southern part of the reserve (south of Four Mile Creek), where the water depth does not reach 20 m, the reserve extends to one Banded morwongs can live for almost 100 years. They are commonly seen on exposed reefs, often congregating in large caves. kilometre offshore. Protected within this reserve, they are heavily targeted outside the A no-take zone exists between Cape Boullanger in the reserve as a live export fish. Photo: Emma Flukes north and Return Point in the south. In this zone, fishing and other extractive activities are prohibited. Things to do Snorkelers can access the reserve in many Getting there locations. A popular snorkel is the Darlington Maria Island is located on Tasmania’s east coast about 8 km jetty. Its pylons are covered in colourful sponges offshore. It is accessible via a 30-45 minute ferry ride or and jewel anemones. Take extra care as power private boat from Triabunna. boats and ferries frequently use this jetty. Triabunna A wide variety of dives are possible in the reserve. One of the more accessible shore dives is the boulder reef north of the jetty Orford where large lobsters, bastard trumpeter, banded Ile du Nord morwong and boarfish are regularly seen. Cape Boullanger Tasmania’s cool water environments can be MARINE RESERVE Darlington challenging for people accustomed to diving in E V R E S E R warmer waters, so seek local advice from dive E N I R A M charter operators. -

Regional Classification of Tasmanian Coastal Waters
REGIONAL CLASSIFICATION OF TASMANIAN COASTAL WATERS AND PRELIMINARY IDENTIFICATION OF REPRESENTATIVE MARINE PROTECTED AREA SITES G.J. Edgar, J. Moverley, D. Peters and C. Reed Ocean Rescue 2000 - Marine Protected Area Program 1993/94 Project No. D705 Report to: Australian Nature Conservation Authority From: Parks and Wildlife Service, Department of Environment & Land Management 134 Macquarie St, Hobart, Tasmania 1 EXECUTIVE SUMMARY Analysis of the distribution of reef plants and animals at over 150 sites around the Tasmanian coastline and Bass Strait islands indicated that Bass Strait reef communities were distinctly different from those occurring further south. This major division in reef ecosystems reflected a boundary near Cape Grim and Little Musselroe Bay between two biogeographical provinces. Each of the two bioprovinces was divisible into four biogeographical regions (bioregions), which occurred along the northern Tasmanian coast and at the Kent Group, Furneaux Group and King Island in Bass Strait, and along the northeastern, southeastern, southern and western coasts of Tasmania. In contrast to these patterns identified using data on coastal reef communities, regional classifications for estuarine and soft-sediment faunas (based on the distribution of beach-washed shells and beach-seined fishes) were less clearly defined. In order to manage and protect Tasmanian inshore plants and animals in accordance with the principle of ecologically sustainable development, an integrated system of representative marine protected areas is considered -

Hobart-Surrounds.Pdf
Lake Fleurieu Pt Wineglass Bay Samuel Woodbury LAKE C Forestier Lake CRESCENT Highland Waters Promise Bay BRADYS FREYCINET LAKE Dee Weatherhead Pt Lagoon TOOMS PENINSULA LAKE L Binney S cho C Degerando Mossy Tungatinah ute Lagoon n P Marsh Lemont assa Pond ge Oatlands Lake Tarraleah Dulverton SCHOUTEN Andover Little Swanport C Faure ISLAND 11 11 C Sonnerat 17 Taillefer Rks Wayatinah 24 Bothwell Jericho Wayatinah Lagoon 21 LAKE Ile des Phoques TIBERIAS 18 Lake 23 15 L Daphne Catagunya 18 30 Melton B31 Woodsdale Lake Ouse Mowbray Repulse B110 17 7 22 Triabunna Cluny Lagoon Kempton Colebrook Levendale 9 12 Hamilton Louisville Meadowbank PROSSER BAY Ile du Nord Lake Orford 20 Dysart 22 23 21 17 Spring Beach Darlington Elderslie 28 Buckland 11 PASSAGE 19 Ellendale Bagdad 17 MOUNT FIELD S E A NATIONAL PARK Fentonbury Broadmarsh Mangalore Runnymede Mistaken Cape Lake 19 Campania Fenton Westerway Gretna 16 Riedle B61 Pontville 14 OYSTER Bay Lake 11 Glenora Tea Tree Dobson 20 20 Lake National Park Rosegarland Brighton 6 BAY Belton Bushy Park 5 Tyenna B62 6 B61 Bridgewater Richmond Pawleena 29 13 16 MERCURY Plenty Hayes 14 9 B10 Granton Maydena 18 Old Beach 13 MARION 17 Sorell New Norfolk B32 Midway 5 B31 Bream BAY Molesworth PITT Pt WATER Forcett 13 21 Creek B35 Cambridge Lewisham Lachlan 12 Copping Marion Bay Mt Lloyd Collinsvale GLENORCHY CLARENCE Dodges 10 Seven Mile Ferry BAY NORTH HOBART Bellerive Beach BAY 11 Carlton Primrose BLACKMAN Rokeby 20 5 Sands Dunalley Fern Tree Lauderdale FORESTIER FREDERICK HENRY RALPHS BAY PENINSULA Mountain -
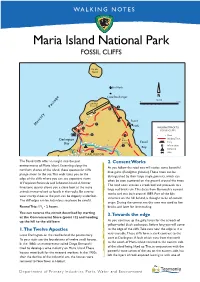
Maria Island National Park FOSSIL CLIFFS
WALKING NOTES Maria Island National Park FOSSIL CLIFFS Ile du Nord Bird Rock Cape Boullanger SCALE 0 1 km Landing Ground 5 6 fencefence 4 Fossil 8 7 Mercury Passage Clis 9 10 WALKING TRACK TO 12 FOSSIL CLIFFS 11 3 Road To Walking Track Darlington Bishop Bay & Clerk Fossil Ferry Information 2 Bay Rangers Camping Station 1 Stile DARLINGTON The Fossil Cliffs offer an insight into the past 2. Cement Works environments of Maria Island. Extending along the As you follow the road you will notice some beautiful northern shores of the island, these spectacular cliffs blue gums (Eucalyptus globulus). These trees can be plunge sheer to the sea. This walk takes you to the distinguished by their large single gumnuts, which can edge of the cliffs where you can see expansive views often be seen scattered on the ground around the trees. of Freycinet Peninsula and Schouten Island. A former The road soon crosses a creek bed and proceeds to a limestone quarry allows you a close look at the many large red brick ruin. This dates from Bernacchi’s cement animals immortalised as fossils in the rocks. Be sure to works and was built around 1889. Part of the kiln wear sturdy shoes as the path can be slippery underfoot. structure on the hill behind, is thought to be of convict The cliff edges can be hazardous so please be careful. origin. During the convict era this area was used to fire 1 Round Trip: 1 /2 - 2 hours. bricks, and later for lime-making. You can reverse the circuit described by starting 3. -

Surrounded by the Clear Temperate Waters Off Maria Island, Charter
Steeped in Human endeavour history and geology have left STORY BY IAN CONNELLAN a fascinating imprint PHOTOGRAPHY BY DON FUCHS on Maria Island EVILLE RANSLEY slowed his boat, MV Cresent, about 500 m from Shoal Bay’s shore, on Maria Island’s western side. A thin strip of glaring white, seaweed-littered beach lay ahead and I asked if we’d slowed down to avoid a sandbar or some other hidden danger. “There’s no problem,” Neville said, gesturing over the side. “In sunny weather the water here’s so clear you can’t Ntell how deep it is.” As Neville cut the engines, his son, Jim, began loading the boat’s tinny with rucksacks for the walking group we were dropping off at McRaes Isthmus, which forms Maria’s narrow waist. Maria’s spine of steep mountains, crowned with clouds, filled the sky to the north-east. To the south-west lay Maria’s rounded hills. It was a warm day and the white sand, blue sky, cloud-shrouded mountains and crystal-clear water seemed more like tropical Queensland than temperate Tasmania. Much of the State is renowned for being cool and wet, but most of its rain – blown from the west on the roaring forties – is scraped from the sky by central ranges before reaching the east coast and Maria. As a result, the island’s often warmer and drier than much of Tasmania – a big attraction. The tinny growled towards the shore with rucksacks, Jim, walker Stuart Spenceley and me. The water in the shallows was almost transparent and, but for our wake, we could have been gliding on wind-riffled glass. -

Australian Convict Sites
167°57'0"E 167°58'0"E 150°E 160°E 170°E New Caledonia 0 0 C 0 6 0 1 o 1 2 ll F 8 0 8 140 in e Bur0 nt Pine s r n 0 10 H y W 1 ad e a 2 o a t 0 R d L e a r te NORFOLK n m 1 a R 2 e i g o l 0 l M0 iddlegate le a C 0 d d ISLAND 1 id AUSTRALIA r d 30°S 30°S e a M 0 e o 1 100 2 20 R Airport k 1 0 s 0 or 1 Runway yl Ta 1 Ferny 0 Lane 0 PACIFIC OCEAN 0 N 0 1 e 80 w d 0 a 120 0 1 o 8 1 0 8 R 0 F 0 0 0 40°S 40°S a 80 8 120 r C 0 l m Cou 8 il 1 n d o 2 a 0 0 0 tr o H TASMAN 80 y R u 1 R n 0 8 6 S 0 o t y S 0 t " 0 r " SEA 8 a 8 y 0 o 0 0 0 0 ' d 8 1 o 2 ' 0 1 NEW ZEALAND 3 R R 3 0 ° o ° 6 9 a 9 2 d 2 1:50,000,000 8 0 d a o R 150°E 160°E 170°E ate 80 leg d d E i ' M 5 5 E 2 ° ° 0 Longridge 7 8 6 6 1 1 k Arthur's Vale ree S S C ° ° C 9 9 o 2 2 un try R o ad 60 80 Q NORFOLK uality Row 40 4 0 d 4 n a 0 Driver C o ISLAND w hristian R o Cemetery T Kingston Cresswell Bay Bay Stree t S S ' ' Kingston Jetty 5 5 Cemetery Bay ° NEPEAN ° 9 9 Slaughter Bay 2 2 PACIFI C ISLAND Emily POINT Bay HUNTER et OCEAN tre Bay S PHILLIP Sydney Bay 1:300,000 ISLAND 1 1 6 6 7 8 ° ° 5 E 5 ' E Kingston and Arthur's Vale S S " Minor roads " Historic Area, Norfolk Island 0 PACIFIC OCEAN 0 ' ' 4 4 ° Contours (20m interval) ° Map produced by 9 9 2 2 Environmental Resources Information Network Convict built channel Australian Government Department of the Convict built structures Environment, Water, Heritage and the Arts Data used © Commonwealth of Australia 2008 Nominated World Heritage area © Map Data Sciences, PSMA Pty Ltd 2007 Reefs and Rock ledges 0 125 250 500 m Land parcels -

Draft Prosser Catchment Plan 2002
PROSSER CATCHMENT MANAGEMENT PLAN DRAFT - June 2002 Prepared for the Glamorgan Spring Bay Landcare Management Committee Funded by the Natural Heritage Trust Prepared by Rob D’Emden BEng, MEngSci, Grad Dip Env Studies GLAMORGAN ••• SPRING BAY COUNCIL TABLE OF CONTENTS GLOSSARY AND ABBREVIATIONS ........................................................................ V FOREWORD ........................................................................................................ 1 EXECUTIVE SUMMARY ....................................................................................... 2 CATCHMENT MANAGEMENT OBJECTIVES AND RECOMMENDED ACTIONS ...... 9 PART 1 BACKGROUND TO THE PLAN ................................................................ 16 1.1 BACKGROUND .......................................................................................................................... 16 1.2 CATCHMENT MISSION , VISION AND STRATEGIES ............................................................... 16 1.3 VALUES AND ISSUES ................................................................................................................ 17 1.4 INTENTION OF THE PLAN ....................................................................................................... 18 1.5 PRINCIPLES OF THE PLAN ....................................................................................................... 18 1.6 RELATIONSHIP TO OTHER MANAGEMENT PLANS ............................................................. 19 1.7 NATURAL RESOURCE MANAGEMENT AND -
Small South-East Islands Draft Management Plan 2002
Small South-East Islands Draft Management Plan July 2002 Small South-East Islands Draft Management Plan 2002 Small South-East Islands Management Plan This draft management plan covers the following small islands of south-east Tasmania: Betsey Island – Nature Reserve Little Betsey Island – Nature Reserve Sloping Island – part of Lime Bay State Reserve Hog Island – part of Lime Bay State Reserve Spectacle Island – Public Reserve Lachlan Island – Unallocated Crown Land Wedge Island – Unallocated Crown Land Isle of Caves – Unallocated Crown Land Little Spectacle Island – Unallocated Crown Land Iron Pot – Unallocated Crown Land Dart Island – Unallocated Crown Land For the four islands reserved under the National Parks and Wildlife Act 1970, this draft management plan has been prepared in accordance with the requirements of Part IV of the Act. With respect to the Public Reserve, the management plan has been prepared in accordance with the Crown Land Act 1976. For the remaining islands unallocated crown land islands, this management plan will be used as a policy guide by the Department of Primary Industries, Water and Environment. Unless otherwise specified, this plan adopts the interpretation of terms given in Section 3 of the National Parks and Wildlife Act 1970. The term ‘Minister’ when used in the plan means the Minister administering the Act. In accordance with Section 23 (2) of the National Parks and Wildlife Act 1970, the managing authority for the reserves, in this case the Director of National Parks and Wildlife, shall carry out his or her duties in relation to the islands for the purpose of giving effect to, and in accordance with, the provisions of, this management plan. -

Island Biology 2019, Third International Conference on Island Ecology, Evolution and Conservation Olivier Flores, Claudine Ah-Peng, Nicholas Wilding
Island Biology 2019, Third International Conference on Island Ecology, Evolution and Conservation Olivier Flores, Claudine Ah-Peng, Nicholas Wilding To cite this version: Olivier Flores, Claudine Ah-Peng, Nicholas Wilding. Island Biology 2019, Third International Con- ference on Island Ecology, Evolution and Conservation: Book of Abstracts. Island Biology 2019, Jul 2019, Saint Denis, France. 2020. hal-02633975v3 HAL Id: hal-02633975 https://hal.univ-reunion.fr/hal-02633975v3 Submitted on 17 Jul 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. BOOK OF ABSTRACTS Université de la Réunion Campus du Moufia 2 Island Biology Third International Conference on Island Ecology, Evolution and Conservation 8-13 July 2019 University of La R´eunion Saint Denis, France Book of Abstracts Editors: Olivier Flores Claudine Ah-Peng Nicholas Wilding Description of contents This document contains the collection of abstracts describing the research works presented at the third international conference on island ecology, evolution and conservation, Island Biology 2019, held in Saint Denis (La R´eunion,8-13 July 2019). In the following order, the different parts of this document concern Plenary sessions, Symposia, Regular sessions, and Poster presentations organized in thematic sections. -

Engaging the Convict Legacy: Art’S Role As a Means of Understanding by Christina Janette Henri, BFA Hons, MFA
Engaging the Convict Legacy: Art’s Role as a Means of Understanding by Christina Janette Henri, BFA Hons, MFA Submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy University of Tasmania November 2011 Statement of Originality This thesis contains no material which has been accepted for a degree or diploma by the University or any other institution, except by way of background information and duly acknowledged in the thesis, and to the best of the my knowledge and belief no material previously published or written by another person except where due acknowledgement is made in the text of the thesis, nor does the thesis contain any material that infringes copyright. Signed: Statement of Authority to Access This thesis may be made available for loan. Copying of any part of this thesis is prohibited for two years from the date this statement was signed; after that time limited copying and communication is permitted in accordance with the Copyright Act 1968 Signed: Abstract Based on the heritage sites that form part of Australia’s convict legacy, focusing mainly within Van Diemen’s Land (Tasmania), this project explores ways in which the economic, social and cultural context of the transportation experience can be communicated to the 21st century visitors to the various sites. The importance of art in mediating site in the service of history will be demonstrated through the conception and curation of a major installation work inviting international participation. Behind the practice-led investigation lies research into possible interpretative and art installation strategies for specific sites: some of the strategies have been initiated in the course of this project, while others remain as suggestions for consideration in future contexts All aspects of the project retain a focus on demonstrating the importance of art as a significant tool to bring attention to history.