Colloids CHAPTER
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Producing Gold Colloids Before the Addition of the Reducing Agent, 100% Gold Ions Exist in Solution
March 1, 2001 Published on IVD Technology (http://www.ivdtechnology.com/article/manufacturing-gold-sol) Manufacturing high-quality gold sol Understanding key engineering aspects of the production of colloidal gold can optimize the quality and stability of gold labeling components. By: Basab Chaudhuri and Syamal Raychaudhuri As early as the first decade of the twentieth century, colloidal gold sols containing particles smaller than 10 nm were being produced by chemical methods. However, these inorganic suspensions were not applied to protein labeling until 1971, when Faulk and Taylor invented the immunogold staining procedure. Since that time, the labeling of targeting molecules, especially proteins, with gold nanoparticles has revolutionized the visualization of cellular and tissue components by electron microscopy. The silver enhancement method has extended the range of application of gold labeling to include light microscopy. The electron-dense and visually dense nature of gold labels also facilitates detection in such techniques as blotting, flow cytometry, and hybridization assays. Double- and triple-labeling systems involving immunogold methods have been used successfully to detect more than one antigen at the same time. The first step toward manufacturing a consistent gold-protein conjugate is to make a gold sol having particles of proper size and dimension. Basically, colloidal gold sols consist of small granules of this transition metal in a stable, uniform dispersion. Most preparations of colloidal gold are made up of particles varying in diameter from about 5 to around 150 nm. For the development of diagnostic assays that make use of gold conjugates, typical particle sizes in the gold sol range between 20 and 40 nm. -

Chemistry Study Materials for Class 9
Chemistry Study Materials for Class 9 ) (NCERT based Revision of Chapter - 2 Ganesh Kumar Date:- 11/10/2020 Is Matter Around Us Pure COLLOIDAL SOLUTIONS A colloidal solution, occasionally identified as a colloidal suspension, is a mixture in which a substances regularly suspended in a fluid. A colloid is a minutely small material that is regularly spread out all through another substance. Properties of a colloid A colloid is a heterogeneous mixture. The size of particles of a colloid is too small to be individually seen by naked eyes. Colloids are big enough to scatter a beam of light passing through it and make its path visible. They do not settle down when left undisturbed, that is, a colloid is quite stable. They cannot be separated from the mixture by the process of filtration. The components of a colloidal solution are the dispersed phase and the dispersion medium. The solute-like component or the dispersed particles in a colloid form the dispersed phase, and the component in which the dispersed phase is suspended is known as the dispersing medium. Colloids are classified according to the state (solid, liquid or gas) of the dispersing medium and the dispersed phase. Colloidal solutions have three sub-classifications: Foams, emulsions and sol. Foam in this setting is created by ensnaring a gas in a liquid. The substance being dispersed would be the gas, triggering the fluid to become frothy and foamy. A sample of this would be shaving cream. An emulsion is a combination of liquids; it’s basically when one liquid is consistently dispersed all through another liquid. -

Surface Chemistry
SURFACE CHEMISTRY Adsorption Adsorption is due to the presence of unbalanced forces, believed to have developed either during crystallisation of solids or due to presence of unpaired, electrons or free valencies in solids having d–orbital. In liquids it is due to surface tension. Difference between adsorption and absorption Sr. No. Adsorption Absorption 1 Adsorption is a surface phenomenon. The adsorbing It is a bulk phenomenon. In substance is called adsorbate and is only concentrated absorption the substance on the surface of adsorbent. penetrates into the bulk of the other substance. 2 The rate of adsorption is rapid to start with and its rate Absorption occurs at a uniform slowly decreases. rate The amount of heat evolved when one mole of an adsorbate gets adsorbed on the surface of an adsorbate is called molar heat (enthalpy) of adsorption. Adsorption in Terms of Gibb’s Helmholtz Equation Adsorption is an exothermic reaction. Therefore, adsorption is accompanied by release of energy or H is always negative and favours the process. Also absorbate molecules get lesser opportunity to move about on the surface of adsorbent. Thus, entropy factor opposes the process. According to Gibb’s Helmholtz equation, G = H – T S Since adsorption does actually take place, H is greater than T S G is negative. As the adsorption continues, the difference between the two opposing tendencies becomes lesser and lesser till they are equal i.e., H = TS or G = 0. At this stage, equilibrium called adsorption equilibrium gets established and there is no net adsorption taking place at this stage. Types of Adsorption There are two types of adsorption. -

Subject: Chemistry Date -11/08/2021 1.What Is a Suspension?
Class: IX Topic: Is matter around us pure? Subject: Chemistry Date -11/08/2021 1.What is a suspension? A suspension is formed when two or more substances are mixed in a non-uniform manner. Heterogeneous mixtures are suspensions. The solute does not mix with the solvent and can be viewed through naked eyes. Properties of Suspensions: • A suspension is a heterogeneous mixture. • We can see the particles of suspensions through naked eyes. • We can see the path of light through the particles of a suspension..This is called Tyndall effect. • The particles of suspension tend to settle down when left undisturbed. Then, they can be separated using filtration. • The size of the particles of solute is more than 1000nm. • The suspension is unstable in nature. 2.What are colloids or colloidal solutions? A colloidal solution or a colloid is a uniform solution of two or more substances. The particles are relatively smaller than suspension .It appears as a homogeneous mixture but it is not. Properties of colloids: • Colloids are heterogeneous in nature. • The particles of a colloid cannot be seen through naked eyes. • The particles scatter a beam of light passed through a colloid and produce Tyndall effect. • Colloids are stable in nature. The particles of colloids do not settle down if left uninterrupted. • We cannot separate the particles of a colloid through filtration. We use a method called Centrifugation to separate the particles of a colloid. • The size of particles of colloid is 1to 1000 nm. 3.What is the Tyndall Effect? When a beam of light is passed through a colloid the particles of the colloid scatter the beam of light and we can see the path of light in the solution. -

Mechanical Dispersion Method: in This Method, the Large Particles of the Material Whose Sol Is to Be Prepared Are Broken Down in a Machine Called Colloid Mill
Arvind Sir Harsh Sir Suri Sir Theory Class: Tuesday Theory Class: Monday Theory Class: & Friday (9pm) & Thursday (9pm) Wednesday & Saturday MCQ Class: Thursday MCQ Class: (9pm) (8pm) Wednesday (8pm) MCQ Class: Monday (8pm) Daily Schedule Learning objectives: Types of colloidal solution. Types of colloidal solution Lyophilic Solution Lyophobic solution Lyophilic Solutions: ➢ Solvent loving ➢ Easy to prepare ➢ Quite stable ( Not easily coagulated) ➢ Reversible in nature Eg. Protein + H2O Starch + H2O Lyophobic Solutions: ➢ Solvent hating ➢ Difficult to prepare ➢ Unstable (easily coagulated) ➢ Irreversible in nature Eg. Sulphur sol + H2O Fe(OH)3 solution + H2O Methods to prepare colloidal solutions: For Lyophilic: It can be prepared easily by just adding hot/cold water Eg. Protein powder + cold water For Lyophobic solutions: It is not easy to prepare. There are some methods to prepare lyophobic colloids. Mechanical dispersion Mechanical dispersion method: In this method, the large particles of the material whose sol is to be prepared are broken down in a machine called colloid mill. Colloid mill consists of two heavy steel discs separated by a little gap. The can be adjusted according to the particle size desired. The two discs rotate at high speed (about 8000 revolutions per minute) in the opposite direction. Notes for reference A suspension of the substance in water is added into the mill. The particles present in the suspension are grinded to form the particles of colloidal size and als get dispersed in water to form a sol. Finer dispersion can be obtained by adding an inert diluents which prevents the colloidal particles to grow in size. For example, glucose is used as diluents in the preparation of sulphur sol. -

Colloid Chemistry
COLLOID CHEMISTRY MD. KHAIRUL ISLAM Md. Khairul Islam, Lecturer, ACCE, R.U. 4/15/2016 HISTORICAL BACKGROUND Thomas Graham (1861) observed that crystalline substances such as sugar, urea, and sodium chloride passed through the membrane, while others like glue, gelatin and gum arabic did not. The former he called crystalloids and the latter colloids (Greek, kolla = glue ; eidos = like). Graham thought that the difference in the behavior of ‘crystalloids’ and ‘colloids’ was due to the particle size. Later it was realised that any substance, regardless of its nature, could be converted into a colloid by subdividing it into particles of colloidal size. 1°A True solution 10ºA Colloidal solution 2000ºA Suspension 2 Md. Khairul Islam, Lecturer, ACCE, R.U. 4/15/2016 DEFINITION o A colloid is a type of chemical mixture in which one substance is dispersed evenly throughout another – homogenous mixture. o The substance distributed as the colloidal particles is called the Dispersed phase. The second continuous phase in which the colloidal particles are dispersed is called the Dispersion medium. 3 Md. Khairul Islam, Lecturer, ACCE, R.U. 4/15/2016 COLLOIDAL SYSTEM CONSISTS OF TWO SEPARATE PHASES 1. a dispersed phase or also known as internal phase (for solute) 2. a continuous phase or dispersion medium (for solvent) A colloidal system may be solid, liquid, or gaseous. 4 Md. Khairul Islam, Lecturer, ACCE, R.U. 4/15/2016 CLASSIFICATION OF COLLOIDS Dispersed Phase Medium / Phases Gas Liquid Solid NONE Liquid Aerosol Solid Aerosol (All gases are Examples: fog, Examples: Gas mutually mist, hair smoke, cloud, miscible) sprays air particulates Emulsion Sol Foam Continuous Examples: milk, Examples: Liquid Example: Medium mayonnaise, pigmented ink, whipped cream hand cream blood Solid Foam Gel Examples: Solid Sol Examples: agar, Solid aerogel, Example: gelatin, jelly, styrofoam, cranberry glass silicagel, opal pumice 5 Md. -
Use of Sol-Gels in the Application of Ceramic Oxide Thin Films Bradley Alan Paulson Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1987 Use of sol-gels in the application of ceramic oxide thin films Bradley Alan Paulson Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Chemical Engineering Commons Recommended Citation Paulson, Bradley Alan, "Use of sol-gels in the application of ceramic oxide thin films " (1987). Retrospective Theses and Dissertations. 11716. https://lib.dr.iastate.edu/rtd/11716 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS While the most advanced technology has been used to photograph and reproduce this manuscript, the quality of the reproduction is heavily dependent upon the quality of the material submitted. For example: • Manuscript pages may have indistinct print. In such cases, the best available copy has been filmed. • Manuscripts may not always be complete. In such cases, a note will indicate that it is not possible to obtain missing pages. • Copyrighted material may have been removed from the manuscript. In such cases, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, and charts) are photographed by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each oversize page is also filmed as one exposure and is available, for an additional charge, as a standard 35mm slide or as a 17"x 23" black and white photographic print. -

Rheology.Pdf
RHEOLOGY Edited by Juan De Vicente Rheology Edited by Juan De Vicente Published by InTech Janeza Trdine 9, 51000 Rijeka, Croatia Copyright © 2012 InTech All chapters are Open Access distributed under the Creative Commons Attribution 3.0 license, which allows users to download, copy and build upon published articles even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. After this work has been published by InTech, authors have the right to republish it, in whole or part, in any publication of which they are the author, and to make other personal use of the work. Any republication, referencing or personal use of the work must explicitly identify the original source. As for readers, this license allows users to download, copy and build upon published chapters even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. Notice Statements and opinions expressed in the chapters are these of the individual contributors and not necessarily those of the editors or publisher. No responsibility is accepted for the accuracy of information contained in the published chapters. The publisher assumes no responsibility for any damage or injury to persons or property arising out of the use of any materials, instructions, methods or ideas contained in the book. Publishing Process Manager Daria Nahtigal Technical Editor Teodora Smiljanic Cover Designer InTech Design Team First published February, 2012 Printed in Croatia A free online edition of this book is available at www.intechopen.com Additional hard copies can be obtained from [email protected] Rheology, Edited by Juan De Vicente p. -

Guide to the Nomenclature of Particle Dispersion Technology for Ceramic Systems
NAT L INST. OF STAND & TECH PUBLICATIONS United States Department of Commerce Technology Administration rviisr National Institute of Standards and Technology NIST Special Publication 945 Guide to the Nomenclature of Particle Dispersion Technology for Ceramic Systems Vincent A. Hackley !57 (0.945 •000 rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and metliods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, -

(1999) the Yield Stress—A Review Or 'Panta Roi'-Everything Flows?
J. Non-Newtonian Fluid Mech., 81 (1999) 133–178 The yield stress—a review or ‘panta roi’—everything flows? Howard A. Barnes Unile6er Research Port Sunlight, Bebington, Wirral, L63 3JW Merseyside, UK Received 30 March 1998; received in revised form 4 May 1998 Abstract An account is given of the development of the idea of a yield stress for solids, soft solids and structured liquids from the beginning of this century to the present time. Originally, it was accepted that the yield stress of a solid was essentially the point at which, when the applied stress was increased, the deforming solid first began to show liquid-like behaviour, i.e. continual deformation. In the same way, the yield stress of a structured liquid was originally seen as the point at which, when decreasing the applied stress, solid-like behaviour was first noticed, i.e. no continual deformation. However as time went on, and experimental capabilities increased, it became clear, first for solids and lately for soft solids and structured liquids, that although there is usually a small range of stress over which the mechanical properties change dramatically (an apparent yield stress), these materials nevertheless show slow but continual steady deformation when stressed for a long time below this level, having shown an initial linear elastic response to the applied stress. At the lowest stresses, this creep behaviour for solids, soft solids and structured liquids can be described by a Newtonian-plateau viscosity. As the stress is increased the flow behaviour usually changes into a power-law dependence of steady-state shear rate on shear stress. -

5 SURFACE CHEMISTRY Word Document 2 .Pdf
STD XII CHEMISTRY CHAPTER 5 Surface Chemistry “The branch of physical chemistry, which deals the Examples :(i) Water vapours Examples : (i) Water vapours nature of surfaces and also with the chemical and physical adsorbed by silica gel. absorbed by anhydrous CaCl2 processes which takes place on the surfaces, is called surface (ii) NH3is adsorbed by charcoal. (ii) NH3is absorbed in water chemistry”. forming NH4OH In surface chemistry, we study the phenomenon of (4) Surface forces :Only the surface atoms of an adsorption, catalysis and colloidal properties. adsorbent play an active role in adsorption. These atoms posses Adsorption unbalanced forces of various types such as, Vander Waal’s forces (1) Definition : The phenomenon of attracting and and chemical bond forces. retaining the molecules of a substance on the surface of a liquid Thus, the residual force-field on a free surface or solid resulting in to higher concentration of the molecules on which is responsible for adsorption is produced. For the surface is called adsorption. example, when a solid substance is broken into two pieces, two (2) Causes of adsorption:Unbalanced forces of new surfaces are formed and therefore, the number of attraction or free valencies which is present at the solid or liquid unbalanced forces becomes more. As a result the tendency for surface, have the property to attract and retain the molecules of adsorption become large. a gas or a dissolved substance on to their surfaces with which (5) Reversible and Irreversible adsorption :The they come in contact. adsorption is reversible, if the adsorbate can be easily removed Example:Ammonia gas placed in contact with charcoal from the surface of the adsorbent by physical methods.If the gets adsorbed on the charcoal whereas ammonia gas placed in adsorbatecan not be easily removed from the surface of the contact with water gets absorbed into water, adsorbent is called irreversible adsorption. -
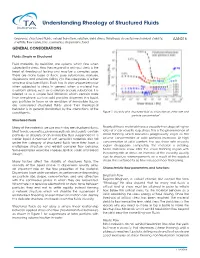
Understanding Rheology of Structured Fluids
Understanding Rheology of Structured Fluids Keywords: structured fluids, sol gel transition, solution, yield stress, thixotropy, viscosity, mechanical stability, AAN016 shelf life, flow curve, inks, cosmetics, dispersions, food GENERAL CONSIDERATIONS Fluids: Simple or Structured Fluid materials, by definition, are systems which flow when subjected to stress. How they respond to an input stress is the heart of rheological testing and may be a complex issue. There are many types of fluids: pure substances, mixtures, dispersions and solutions, falling into the categories of either simple or structured fluids. Each has its own unique behavior when subjected to stress. In general, when a material has a uniform phase, such as a solution or pure substance, it is referred to as a simple fluid. Materials which contain more than one phase, such as solid particles dispersed in a liquid, gas particles in foam or an emulsion of immiscible liquids are considered structured fluids since their rheological behavior is in general dominated by the interactions of the constituents. Figure 1: Viscosity of a structured fluid as a function of shear rate and particle concentration1 Structured fluids Many of the materials we use each day are structured fluids. Nearly all these materials have a viscosity that drops at higher Most foods, cosmetics, pharmaceuticals and paints contain rates of shear velocity resp, stress. This is the phenomenon of particles or droplets of an immiscible fluid suspended in a shear thinning which becomes progressively larger as the carrier liquid. A number of soft semisolid materials also fall volume concentration of solid particles increases. At high under the category of structured fluids since they have a concentration of solid content, the low shear rate viscosity multiphase structure and exhibit complex flow behavior.