New Psychoactive Substances (NPS)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recommended Methods for the Identification and Analysis of Synthetic Cathinones in Seized Materialsd
Recommended methods for the Identification and Analysis of Synthetic Cathinones in Seized Materials (Revised and updated) MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES Photo credits:UNODC Photo Library; UNODC/Ioulia Kondratovitch; Alessandro Scotti. Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Recommended Methods for the Identification and Analysis of Synthetic Cathinones in Seized Materials (Revised and updated) MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES UNITED NATIONS Vienna, 2020 Note Operating and experimental conditions are reproduced from the original reference materials, including unpublished methods, validated and used in selected national laboratories as per the list of references. A number of alternative conditions and substitution of named commercial products may provide comparable results in many cases. However, any modification has to be validated before it is integrated into laboratory routines. ST/NAR/49/REV.1 Original language: English © United Nations, March 2020. All rights reserved, worldwide. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of names of firms and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. Acknowledgements The Laboratory and Scientific Section of the UNODC (LSS, headed by Dr. Justice Tettey) wishes to express its appreciation and thanks to Dr. -

Synthetic Cannabinoids-1) IMMUNALYSIS DIRECT ELISA Kit for Forensic Matrices
K2 (Synthetic IMMUNALYSIS Cannabinoids-1) DIRECT ELISA Kit for forensic matrices JWH-018 Demonstrates significant cross reactivity with the major metabolites of JWH-018, JWH-073, Schedule I Controlled Substances: JWH-018, JWH-073, JWH-200, JWH-019, JWH-200 and AM-2201. JWH-122, JWH-398, JWH-081, JWH-250, JWH-203 CP-47,497, CP-47,497 C8, HU-210, HU-211, AM-2201, AM-694, RCS-4 (SR-19), RCS-8 (SR-18) Street Names: Spice, K2, Genie, Yucatan Fire, Skunk, Sence, Smoke, ChillX, Highdi’s Almdröhner, Earth Impact, Gorillaz, Galaxy Gold, Space Truckin, Solar Flare, Moon Rocks, Blue Lotus, Aroma, Scope, Sky, OG Potpourri, Bliss, Black Momba, Bombay Blue, Fake Weed, and Zohai. Urine About Synthetic Cannabinoids: Spice or K2 is a mixture of herbs and spices treated with synthetic compounds similar to THC that is typically sold in head shops, tobacco shops or over the internet. Though not structurally related, K2 mimics the psychoactive stimulant properties of THC but can be 100 to 800 times more potent than THC.1 Administration: Synthetic Cannabinoid products are usually smoked in joints or pipes, and sometimes made into tea.2 Effects: Psychological effects are similar to those of marijuana and include paranoia, panic attacks and giddiness. Physiological effects include increased heart rate and high blood pressure. Long-term effects are not known.2 1. Devane, W. A. et al. A novel probe for the cannabi- noid receptor. Journal of Medical Chemistry 35 (11): 2065–2069 (1992). 2. Drug Enforcement Administration; www.dea.gov. Tel 909.482.0840 | Toll Free -

Modafinil and Modafinil Analogues: Free Radical Mechanism of the Eugeroic and Cognitive Enhancment Effect Clifford Fong
Modafinil and modafinil analogues: free radical mechanism of the eugeroic and cognitive enhancment effect Clifford Fong To cite this version: Clifford Fong. Modafinil and modafinil analogues: free radical mechanism of the eugeroic and cognitive enhancment effect. [Research Report] Eigenenergy. 2018. hal-01933737 HAL Id: hal-01933737 https://hal.archives-ouvertes.fr/hal-01933737 Submitted on 24 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Modafinil and modafinil analogues: free radical mechanism of the eugeroic and cognitive enhancment effect Clifford W. Fong Eigenenergy, Adelaide, South Australia. Keywords: Modafinil, modafinil-like analogues, eugeroic effect, cognitive enhancement, free radicals, quantum mechanics Abbreviations Dopamine DA, dopamine transporter DAT, Dissociative electron transfer or attachment DET, Linear free energy relationship LFER, free energy of water desolvation ΔG desolv,CDS , lipophilicity free energy ΔG lipo,CDS, cavity dispersion solvent structure of the first solvation shell CDS, highest occupied molecular orbital HOMO, lowest unoccupied molecular orbital LUMO, multiple correlation coefficient R 2, the F test of significance, standards errors for the estimate (SEE) and standard errors of the variables SE(ΔG desolCDS ), SE(ΔG lipoCDS ), SE(Dipole Moment), SE (Molecular Volume), transition state TS, reactive oxygen species ROS. -

Euphoric Non-Fentanil Novel Synthetic Opioids on the Illicit Drugs Market
Forensic Toxicology (2019) 37:1–16 https://doi.org/10.1007/s11419-018-0454-5 REVIEW ARTICLE The search for the “next” euphoric non‑fentanil novel synthetic opioids on the illicit drugs market: current status and horizon scanning Kirti Kumari Sharma1,2 · Tim G. Hales3 · Vaidya Jayathirtha Rao1,2 · Niamh NicDaeid4,5 · Craig McKenzie4 Received: 7 August 2018 / Accepted: 11 November 2018 / Published online: 28 November 2018 © The Author(s) 2018 Abstract Purpose A detailed review on the chemistry and pharmacology of non-fentanil novel synthetic opioid receptor agonists, particularly N-substituted benzamides and acetamides (known colloquially as U-drugs) and 4-aminocyclohexanols, developed at the Upjohn Company in the 1970s and 1980s is presented. Method Peer-reviewed literature, patents, professional literature, data from international early warning systems and drug user fora discussion threads have been used to track their emergence as substances of abuse. Results In terms of impact on drug markets, prevalence and harm, the most signifcant compound of this class to date has been U-47700 (trans-3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide), reported by users to give short- lasting euphoric efects and a desire to re-dose. Since U-47700 was internationally controlled in 2017, a range of related compounds with similar chemical structures, adapted from the original patented compounds, have appeared on the illicit drugs market. Interest in a structurally unrelated opioid developed by the Upjohn Company and now known as BDPC/bromadol appears to be increasing and should be closely monitored. Conclusions International early warning systems are an essential part of tracking emerging psychoactive substances and allow responsive action to be taken to facilitate the gathering of relevant data for detailed risk assessments. -

Programme & Abstracts
The 57th Annual Meeting of the International Association of Forensic Toxicologists. 2nd - 6th September 2019 BIRMINGHAM, UK The ICC Birmingham Broad Street, Birmingham B1 2EA Programme & Abstracts 1 Thank You to our Sponsors PlatinUm Gold Silver Bronze 2 3 Contents Welcome message 5 Committees 6 General information 7 iCC maps 8 exhibitors list 10 Exhibition Hall 11 Social Programme 14 opening Ceremony 15 Schedule 16 Oral Programme MONDAY 2 September 19 TUESDAY 3 September 21 THURSDAY 5 September 28 FRIDAY 6 September 35 vendor Seminars 42 Posters 46 oral abstracts 82 Poster abstracts 178 4 Welcome Message It is our great pleasure to welcome you to TIAFT Gala Dinner at the ICC on Friday evening. On the accompanying pages you will see a strong the UK for the 57th Annual Meeting of scientific agenda relevant to modern toxicology and we The International Association of Forensic thank all those who submitted an abstract and the Toxicologists Scientific Committees for making the scientific programme (TIAFT) between 2nd and 6th a success. Starting with a large Young Scientists September 2019. Symposium and Dr Yoo Memorial plenary lecture by Prof Tony Moffat on Monday, there are oral session topics in It has been decades since the Annual Meeting has taken Clinical & Post-Mortem Toxicology on Tuesday, place in the country where TIAFT was founded over 50 years Human Behaviour Toxicology & Drug-Facilitated Crime on ago. The meeting is supported by LTG (London Toxicology Thursday and Toxicology in Sport, New Innovations and Group) and the UKIAFT (UK & Ireland Association of Novel Research & Employment/Occupational Toxicology Forensic Toxicologists) and we thank all our exhibitors and on Friday. -

Plant-Mediated Enantioselective Transformation of Indan-1-One and Indan-1-Ol. Part 2
molecules Article Plant-Mediated Enantioselective Transformation of Indan-1-one and Indan-1-ol. Part 2 Wanda M ˛aczka 1,* , Katarzyna Wi ´nska 1,* , Małgorzata Grabarczyk 1,* and Renata Galek 2 1 Department of Chemistry, Wroclaw University of Environmental and Life Sciences, Norwida 25, 50-375 Wroclaw, Poland 2 Department of Genetics, Plant Breeding and Seed Production, Wroclaw University of Environmental and Life Sciences Pl. Grunwaldzki 24A, 53-363 Wroclaw, Poland; [email protected] * Correspondence: [email protected] (W.M.); [email protected] (K.W.); [email protected] (M.G.); Tel.: +48-71-320-5213 (W.M. & K.W.) Received: 31 October 2019; Accepted: 25 November 2019; Published: 27 November 2019 Abstract: The main purpose of this publication was to obtain the S-enantiomer of indan-1-ol with high enantiomeric excess and satisfactory yield. In our research, we used carrot callus cultures (Daucus carota L.), whereby the enzymatic system reduced indan-1-one and oxidized indan-1-ol. During the reaction of reduction, after five days, we received over 50% conversion, with the enantiomeric excess of the formed S-alcohol above 99%. In turn, during the oxidation of racemic indan-1-ol after 15 days, 36.7% of alcohol with an enantiomeric excess 57.4% S(+) remained in the reaction mixture. In addition, our research confirmed that the reactions of reduction and oxidation are competing reactions during the transformation of indan-1-ol and indan-1-one in carrot callus cultures. Keywords: indan-1-one; indan-1-ol; biotransformation; reduction; oxidation; carrot; callus culture 1. -

Recommended Methods for the Identification and Analysis of Synthetic Cannabinoid Receptor Agonists in Seized Materials (Revised and Updated)
Recommended methods for the Identification and Analysis of Synthetic Cannabinoid Receptor Agonists in Seized Materials (Revised and updated) MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES Photo credits: UNODC Photo Library; UNODC/Ioulia Kondratovitch; Alessandro Scotti. Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Recommended Methods for the Identification and Analysis of Synthetic Cannabinoid Receptor Agonists in Seized Materials (Revised and updated) MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES UNITED NATIONS Vienna, 2020 Note Operating and experimental conditions are reproduced from the original reference materials, including unpublished methods, validated and used in selected national laboratories as per the list of references. A number of alternative conditions and substitution of named commercial products may provide comparable results in many cases, but any modification has to be validated before it is integrated into laboratory routines. Mention of names of firms and commercial products does not imply theendorsement of the United Nations. ST/NAR/48/REV.1 © United Nations, July 2020. All rights reserved, worldwide The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. Acknowledgements The Laboratory and Scientific Services of the United Nations Office on Drugs and Crime (UNODC) (LSS, headed by Dr. -

Moves to Amend HF No. 2711 As Follows
05/05/20 REVISOR KLL/JK A20-0767 1.1 .................... moves to amend H.F. No. 2711 as follows: 1.2 Delete everything after the enacting clause and insert: 1.3 "ARTICLE 1 1.4 APPROPRIATIONS 1.5 Section 1. APPROPRIATIONS. 1.6 The sums shown in the column under "APPROPRIATIONS" are added to or reduce the 1.7 appropriations in Laws 2019, First Special Session chapter 5, to the agencies and for the 1.8 purposes specified in this article. The appropriations are from the general fund, or another 1.9 named fund, and are available for the fiscal year indicated for each purpose. 1.10 APPROPRIATIONS 1.11 Available for the Year 1.12 Ending June 30 1.13 2020 2021 1.14 Sec. 2. CORRECTIONS 1.15 Subdivision 1. Total Appropriation $ 205,000 $ 5,545,000 1.16 The amounts that may be spent for each 1.17 purpose are specified in the following 1.18 subdivisions. 1.19 Subd. 2. Correctional Institutions -0- (2,545,000) 1.20 To account for overall bed impact savings of 1.21 reductions in the penalties for controlled 1.22 substances offenses involving the possession 1.23 of marijuana, investments in community 1.24 supervision, and increased penalties for sex 1.25 trafficking offenses, the fiscal year 2021 Article 1 Sec. 2. 1 05/05/20 REVISOR KLL/JK A20-0767 2.1 appropriation from Laws 2019, First Special 2.2 Session chapter 5, article 1, section 15, 2.3 subdivision 2, is reduced by $2,545,000. 2.4 Subd. -
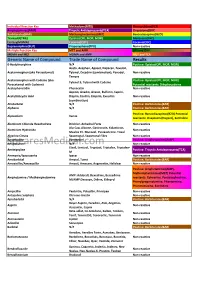
Cross Reaction Guide
Individual Reaction Key Methadone(MTD) Phencyclidine(PCP) Amphetamines(AMP) Tricyclic Antidepressants(TCA) Oxycodone(OXY) Barbiturates(BAR) Methamphetamines(MET) Benzodiazepines(BZO) Tramadol(TML) Opiates(OPI, MOP, MOR) Marijuana(THC) Ecstasy(MDMA) Cotinine(COT) Cocaine(COC) Buprenorphine(BUP) Propoxyphene(PPX) Non-reactive Multiple Reaction Key MET and AMP OPI and OXY MDMA and MET MDMA and AMP MET and TCA Generic Name of Compound Trade Name of Compound Results 6-Acetylmorphine N/A Positive: Opiates(OPI, MOP, MOR) Aceta, Acephen, Apacet, Dapacen, Feverall, Acetaminophen (aka Paracetamol) Tylenol, Excedrin (combination), Panadol, Non-reactive Tempra Acetaminophen with Codeine (aka Positive: Opiates(OPI, MOP, MOR) Tylenol 3, Tylenol with Codeine Paracetamol with Codeine) Potential reactants: Dihydrocodeine Acetophenetidin Phenacetin Non-reactive Aspirin, Anadin, Anasin, Bufferin, Caprin, Acetylsalicyclic Acid Disprin, Ecotrin, Empirin, Excedrin Non-reactive (combination) Allobarbital N/A Positive: Barbiturates(BAR) Alphenol N/A Positive: Barbiturates(BAR) Positive: Benzodiazepines(BZO) Potential Alprazolam Xanax reactants: Oxaprozin (Daypro), Sertraline Aluminum Chloride Hexahydrate Drichlor, Anhydrol Forte Non-reactive Alu-Cap, Alisone, Gastrocote, Kolanticon, Aluminum Hydroxide Non-reactive Maalox TC, Mucogel, Pyrogastrone, Topal Alverine Citrate Spasmonal, Spasmonal Fibre Non-reactive Amantadine Symmetrel Positive: Amphetamines(AMP) SpearesMedical.comAminopyrine N/A Non-reactive Elavil, Lentizol, Tryptizol, Triptafen, Triptafen- Amitriptyline -

References to Argentina
References to Argentina Part 1 RECENT STATISTICS AND TREND ANALYSIS OF ILLICIT DRUG MARKETS A. EXTENT OF ILLICIT DRUG USE AND HEALTH CONSEQUENCES The Americas (pages 12 to 14 WDR 13) In the Americas, a high prevalence of most illicit drugs, essentially driven by estimates in North America, was observed, with the prevalence of cannabis (7.9 per cent) and cocaine (1.3 per cent) being particularly high in the region. South America, Central America and the Caribbean The annual prevalence of cocaine use in South America (1.3 per cent of the adult population) is comparable to levels in North America, while it remains much higher than the global average in Central America (0.6 per cent) and the Caribbean (0.7 per cent). Cocaine use has increased significantly in Brazil, Costa Rica and, to lesser extent, Peru while no change in its use was reported in Argentina. The use of cannabis in South America is higher (5.7 per cent) than the global average, but lower in Central America and Caribbean (2.6 and 2.8 per cent respectively). In South America and Central America the use of opioids (0.3 and 0.2 per cent, respectively) and Ecstasy (0.1 per cent each) also remain well below the global average. While opiates use remains low, countries such as Colombia report that heroin use is becoming increasingly common among certain age groups and socio-economic classes.30 Part 2 NEW PSYCHOACTIVE SUBSTANCES C. THE RECENT EMERGENCE AND SPREAD OF NEW PSYCHOACTIVE SUBSTANCES (page 67 WDR 13) Spread at the global level Number of countries reporting the emergence of new psychoactive substances Pursuant to Commission on Narcotic Drugs resolution 55/1, entitled “Promoting international cooperation in responding to the challenges posed by new psychoactive substances”, in 2012 UNODC sent a questionnaire on NPS to all Member States, to which 80 countries and territories replied. -

The Enantioselective-Controlled Delivery of Propranolol by Cellulose
The Enantioselective-Controlled Delivery of Propranolol by Cellulose Membrane Grafted with Molecularly Imprinted Polymer Chatchada Bodhibukkana A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Pharmaceutical Sciences Prince of Songkla University 2008 i Copyright of Prince of Songkla University Thesis Title The Enantioselective-Controlled Delivery of Propranolol by Cellulose Membrane Grafted with Molecularly Imprinted Polymer Author Miss Chatchada Bodhibukkana Major program Pharmaceutical Sciences Major Advisor Examining Committee ………………………………………… ………………………………Chairperson (Assoc. Prof. Dr. Roongnapa Srichana) (Assoc. Prof. Dr. Ruedeekorn Wiwattanapatapee) ………………………………Committee Co-advisor (Assoc. Prof. Dr. Varaporn Junyaprasert ) ………………………………………… ………………………………Committee (Assoc. Prof. Dr. Teerapol Srichana) (Assoc. Prof. Dr. Roongnapa Srichana) ………………………………………… ………………………………Committee (Prof. Gary P. Martin) (Assoc. Prof. Dr. Teerapol Srichana) The Graduate School, Prince of Songkla University, has approved this thesis as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Pharmaceutical Sciences …………………………………………… (Assoc. Prof. Dr. Krerkchai Thongnoo) Dean of Graduate School ii propranolol !" #$ %& '(")* %&% **+, - )!,!. */*- 0 1,!2 ( 2550 ! 26 7 %8%),(9-+:%&;*,< + (composite membrane) #$ ; :%%* %&% **+, (Molecularly imprinted polymer, MIP) *& !" L* %&L* ; ; S-enantiomer ; racemic propranolol + ,: N68 +%& +N$'O !,& %&$*- #$ ") -

Synthetic Cannabinoids (60 Substances) A) Classical Cannabinoid
Synthetic cannabinoids (60 substances) a) Classical cannabinoid OH H OH H O Common name Chemical name CAS number Molecular Formula HU-210 3-(1,1’-dimethylheptyl)-6aR,7,10,10aR-tetrahydro-1- Synonym: 112830-95-2 C H O hydroxy-6,6-dimethyl-6H-dibenzo[b,d]pyran-9-methanol 25 38 3 11-Hydroxy-Δ-8-THC-DMH b) Nonclassical cannabinoids OH OH R2 R3 R4 R1 CAS Molecular Common name Chemical name R1 R2 R3 R4 number Formula rel-2[(1 S,3 R)-3- hydroxycyclohexyl]- 5- (2- methyloctan- 2- yl) CP-47,497 70434-82-1 C H O CH H H H phenol 21 34 2 3 rel-2[(1 S,3 R)-3- hydroxycyclohexyl]- 5- (2- methylheptan- 2- yl) CP-47,497-C6 - C H O H H H H phenol 20 32 2 CP-47,497-C8 rel-2- [(1 S,3 R)-3- hydroxycyclohexyl]- 5- (2- methylnonan- 2- yl) 70434-92-3 C H O C H H H H Synonym: Cannabicyclohexanol phenol 22 36 2 2 5 CAS Molecular Common name Chemical name R1 R2 R3 R4 number Formula rel-2[(1 S,3 R)-3- hydroxycyclohexyl]- 5- (2- methyldecan- 2- yl) CP-47,497-C9 - C H O C H H H H phenol 23 38 2 3 7 rel-2- ((1 R,2 R,5 R)-5- hydroxy- 2- (3- hydroxypropyl)cyclohexyl)- 3-hydroxy CP-55,940 83003-12-7 C H O CH H H 5-(2- methyloctan- 2- yl)phenol 24 40 3 3 propyl rel-2- [(1 S,3 R)-3- hydroxy-5,5-dimethylcyclohexyl]- 5- (2- Dimethyl CP-47,497-C8 - C H O C H CH CH H methylnonan-2- yl)phenol 24 40 2 2 5 3 3 c) Aminoalkylindoles i) Naphthoylindoles 1' R R3' R2' O N CAS Molecular Common name Chemical name R1’ R2’ R3’ number Formula [1-[(1- methyl- 2- piperidinyl)methyl]- 1 H-indol- 3- yl]- 1- 1-methyl-2- AM-1220 137642-54-7 C H N O H H naphthalenyl-methanone 26 26 2 piperidinyl