Somerset Ecological Networks Report 2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Trunk Road Estate Biodiversity Action Plan
Home Welsh Assembly Government Trunk Road Estate Biodiversity Action Plan 2004-2014 If you have any comments on this document, its contents, or its links to other sites, please send them by post to: Environmental Science Advisor, Transport Directorate, Welsh Assembly Government, Cathays Park, Cardiff CF10 3NQ or by email to [email protected] The same contact point can be used to report sightings of wildlife relating to the Trunk Road and Motorway network. Prepared by on behalf of the Welsh Assembly Government ISBN 0 7504 3243 8 JANUARY 2004 ©Crown copyright 2004 Home Contents Foreword by Minister for Economic Development and Transport 4 Executive Summary 5 How to use this document 8 Introduction 9 Background to biodiversity in the UK 10 Background to biodiversity in Wales 12 The Trunk Road Estate 13 Existing guidance and advice 16 TREBAP development 19 Delivery 23 Links to other organisations 26 The Plans 27 Glossary 129 Bibliography and useful references 134 Other references 138 Acknowledgements 139 3 Contents Foreword FOREWORD BY THE MINISTER FOR ECONOMIC DEVELOPMENT AND TRANSPORT The publication of this Action Plan is both a recognition of the way the Assembly Government has been taking forward biodiversity and an opportunity for the Transport Directorate to continue to contribute to the wealth of biodiversity that occurs in Wales. Getting the right balance between the needs of our society for road-based transport, and the effects of the Assembly’s road network on our wildlife is a complex and often controversial issue. The Plan itself is designed to both challenge and inspire those who work with the Directorate on the National Assembly’s road network – and, as importantly, to challenge those of us who use the network to think more about the wildlife there. -

(Hydrilla Verticillata) Stem Quality
BIOLOGICAL CONTROL 8, 52–57 (1997) ARTICLE NO. BC960484 Growth and Development of the Biological Control Agent Bagous hydrillae as Influenced by Hydrilla (Hydrilla verticillata) Stem Quality G. S. WHEELER AND T. D. CENTER USDA/ARS Aquatic Weed Research Unit, 3205 College Avenue, Ft. Lauderdale, Florida 33314 Received March 11, 1996; accepted August 28, 1996 that reduces the impact of insects imported for weed Plant quality of dioecious hydrilla was studied as a biological control. factor that may influence larval survival, growth, and The Australian weevil Bagous hydrillae O’Brien (Bal- development of the biological control agent Bagous ciunas and Purcell, 1991) was introduced into the hydrillae. Nitrogen content and stem toughness of United States for biological control of hydrilla. Release hydrilla varied among the five sites studied and be- of this species began in 1991, and to date, at least two tween summer and fall collections. The nitrogen con- field populations have established, one in Florida and tent of hydrilla collected during summer ranged from another in Texas (Center et al., unpublished data). 1.2 to 3.6% (dry weight) and during fall from 1.6 to 2.9%. Considerable difficulty has been experienced in estab- Stem toughness ranged from 487 to 940 g/mm2 during lishing this species despite release of several thousand the summer and from 418 to 1442 g/mm2 during the fall. individuals throughout the area. Among the factors The larvae of this weevil species required more time to that could influence weevil performance and establish- complete development when fed hydrilla containing ment, the quality of hydrilla, which varies greatly at lower levels of nitrogen and tougher stemmed plants. -

Water Beetles
Ireland Red List No. 1 Water beetles Ireland Red List No. 1: Water beetles G.N. Foster1, B.H. Nelson2 & Á. O Connor3 1 3 Eglinton Terrace, Ayr KA7 1JJ 2 Department of Natural Sciences, National Museums Northern Ireland 3 National Parks & Wildlife Service, Department of Environment, Heritage & Local Government Citation: Foster, G. N., Nelson, B. H. & O Connor, Á. (2009) Ireland Red List No. 1 – Water beetles. National Parks and Wildlife Service, Department of Environment, Heritage and Local Government, Dublin, Ireland. Cover images from top: Dryops similaris (© Roy Anderson); Gyrinus urinator, Hygrotus decoratus, Berosus signaticollis & Platambus maculatus (all © Jonty Denton) Ireland Red List Series Editors: N. Kingston & F. Marnell © National Parks and Wildlife Service 2009 ISSN 2009‐2016 Red list of Irish Water beetles 2009 ____________________________ CONTENTS ACKNOWLEDGEMENTS .................................................................................................................................... 1 EXECUTIVE SUMMARY...................................................................................................................................... 2 INTRODUCTION................................................................................................................................................ 3 NOMENCLATURE AND THE IRISH CHECKLIST................................................................................................ 3 COVERAGE ....................................................................................................................................................... -

Columbia County Ground Beetle Species (There May Be Some Dutchess County Floodplain Forest Records Still Included)
Columbia County Ground Beetle Species (There may be some Dutchess County floodplain forest records still included). Anisodactylus nigerrimus Amara aenea Apristus latens Acupalpus canadensis Amara angustata Apristus subsulcatus Acupalpus partiarius Amara angustatoides Asaphidion curtum Acupalpus pauperculus Amara apricaria Badister neopulchellus Acupalpus pumilus Amara avida Badister notatus Acupalpus rectangulus Amara chalcea Badister ocularis Agonum aeruginosum Amara communis Badister transversus Agonum affine Amara crassispina Bembidion Agonum canadense Amara cupreolata Bembidion aenulum Agonum corvus Amara exarata Bembidion affine Agonum cupripenne Amara familiaris Bembidion antiquum Agonum errans Amara flebilis Bembidion basicorne Agonum extensicolle Amara lunicollis Bembidion carolinense Agonum ferreum Amara neoscotica Bembidion castor Agonum fidele Amara otiosa Bembidion chalceum Agonum galvestonicum Amara ovata Bembidion cheyennense Agonum gratiosum Amara pennsylvanica Bembidion frontale Agonum harrisii Amara rubrica Bembidion immaturum Agonum lutulentum Amara sp Bembidion impotens Agonum melanarium Amphasia interstitialis Bembidion inaequale Agonum metallescens Anatrichis minuta Bembidion incrematum Agonum moerens Anisodactylus discoideus Bembidion inequale Agonum muelleri Anisodactylus harrisii Bembidion lacunarium Agonum mutatum Anisodactylus kirbyi Bembidion levetei Agonum palustre Anisodactylus nigrita Bembidion louisella Agonum picicornoides Anisodactylus pseudagricola Bembidion mimus Agonum propinquum Anisodactylus rusticus -
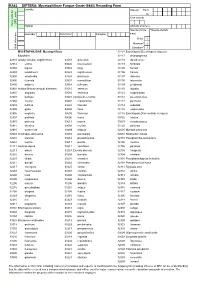
Ra82 Diptera
RA82 DIPTERA: Mycetophilinae Fungus Gnats (6480) Recording Form Locality Date(s) from: to: Vice county GPS users Grey cells for Habitat Altitude (metres) Source (circle *Source details Recorder Determiner Compiler one) Field 1 Museum* 2 Grid reference Literature* 3 MYCETOPHILIDAE: Mycetophilinae 33101 Exechiopsis (Exechiopsis) clypeata Exechiini 33117 dryaspagensis 32801 Allodia (Allodia) anglofennica 33519 griseolum33118 dumitrescae 32912 embla 33526 intermedium33119 fimbriata 32802 lugens 33522 kingi33120 furcata 32803 lundstroemi 33523 nigrofuscum33106 hammi 32804 ornaticollis 33524 proximum33107 indecisa 32806 truncata 33527 rosmellitum33108 intersecta 32805 zaitzevi 33514 ruficorne33109 jenkinsoni 32901 Allodia (Brachycampta) alternans 33515 serenum33110 ligulata 32910 angulata 33516 sericoma33121 magnicauda 32903 barbata 33601 Cordyla brevicornis33112 pseudindecisa 32904 czernyi 33602 crassicornis33113 pulchella 32909 foliifera 33603 fasciata33114 subulata 32905 grata 33604 fissa33115 unguiculata 32906 neglecta 33605 flaviceps33116 Exechiopsis (Xenexechia) crucigera 32907 pistillata 33606 fusca33002 leptura 32915 protenta 33613 insons33003 membranacea 32914 silvatica 33608 murina33122 pollicata 32916 westerholti 33609 nitidula32605 Myrosia maculosa 32602 Allodiopsis domestica 33610 parvipalpis32601 Notolopha cristata 32610 korolevi 33614 pseudomurina32701 Pseudexechia aurivernica 32607 rustica 33611 pusilla32706 monica 32212 Anatella alpina 33612 semiflava32705 parallela 32213 ankeli 33201 Exechia bicincta32703 trisignata 32216 bremia -

Supplementary Material
Alcedo atthis (Common Kingfisher) European Red List of Birds Supplementary Material The European Union (EU27) Red List assessments were based principally on the official data reported by EU Member States to the European Commission under Article 12 of the Birds Directive in 2013-14. For the European Red List assessments, similar data were sourced from BirdLife Partners and other collaborating experts in other European countries and territories. For more information, see BirdLife International (2015). Contents Reported national population sizes and trends p. 2 Trend maps of reported national population data p. 4 Sources of reported national population data p. 6 Species factsheet bibliography p. 11 Recommended citation BirdLife International (2015) European Red List of Birds. Luxembourg: Office for Official Publications of the European Communities. Further information http://www.birdlife.org/datazone/info/euroredlist http://www.birdlife.org/europe-and-central-asia/european-red-list-birds-0 http://www.iucnredlist.org/initiatives/europe http://ec.europa.eu/environment/nature/conservation/species/redlist/ Data requests and feedback To request access to these data in electronic format, provide new information, correct any errors or provide feedback, please email [email protected]. THE IUCN RED LIST OF THREATENED SPECIES™ BirdLife International (2015) European Red List of Birds Alcedo atthis (Common Kingfisher) Table 1. Reported national breeding population size and trends in Europe1. Country (or Population estimate Short-term population trend4 -

Lessons from Genome Skimming of Arthropod-Preserving Ethanol Benjamin Linard, P
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archive Ouverte en Sciences de l'Information et de la Communication Lessons from genome skimming of arthropod-preserving ethanol Benjamin Linard, P. Arribas, C. Andújar, A. Crampton-Platt, A. P. Vogler To cite this version: Benjamin Linard, P. Arribas, C. Andújar, A. Crampton-Platt, A. P. Vogler. Lessons from genome skimming of arthropod-preserving ethanol. Molecular Ecology Resources, Wiley/Blackwell, 2016, 16 (6), pp.1365-1377. 10.1111/1755-0998.12539. hal-01636888 HAL Id: hal-01636888 https://hal.archives-ouvertes.fr/hal-01636888 Submitted on 17 Jan 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 Lessons from genome skimming of arthropod-preserving 2 ethanol 3 Linard B.*1,4, Arribas P.*1,2,5, Andújar C.1,2, Crampton-Platt A.1,3, Vogler A.P. 1,2 4 5 1 Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 6 5BD, UK, 7 2 Department of Life Sciences, Imperial College London, Silwood Park Campus, Ascot 8 SL5 7PY, UK, 9 3 Department -

“Two-Tailed” Baetidae of Ohio January 2013
Ohio EPA Larval Key for the “two-tailed” Baetidae of Ohio January 2013 Larval Key for the “two-tailed” Baetidae of Ohio For additional keys and descriptions see: Ide (1937), Provonsha and McCafferty (1982), McCafferty and Waltz (1990), Lugo-Ortiz and McCafferty (1998), McCafferty and Waltz (1998), Wiersema (2000), McCafferty et al. (2005) and McCafferty et al. (2009). 1. Forecoxae with filamentous gill (may be very small), gills usually with dark clouding, cerci without dark band near middle, claws with a smaller second row of teeth. .............................. ............................................................................................................... Heterocloeon (H.) sp. (Two species, H. curiosum (McDunnough) and H. frivolum (McDunnough), are reported from Ohio, however, the larger hind wing pads used by Morihara and McCafferty (1979) to distinguish H. frivolum have not been verified by OEPA.) Figures from Ide, 1937. Figures from Müller-Liebenau, 1974. 1'. Forecoxae without filamentous gill, other characters variable. .............................................. 2 2. Cerci with alternating pale and dark bands down its entire length, body dorsoventrally flattened, gills with a dark clouded area, hind wing pads greatly reduced. ............................... ......................................................................................... Acentrella parvula (McDunnough) Figure from Ide, 1937. Figure from Wiersema, 2000. 2'. Cerci without alternating pale and dark bands, other characters variable. ............................ -

Superior National Forest
Admirals & Relatives Subfamily Limenitidinae Skippers Family Hesperiidae £ Viceroy Limenitis archippus Spread-wing Skippers Subfamily Pyrginae £ Silver-spotted Skipper Epargyreus clarus £ Dreamy Duskywing Erynnis icelus £ Juvenal’s Duskywing Erynnis juvenalis £ Northern Cloudywing Thorybes pylades Butterflies of the £ White Admiral Limenitis arthemis arthemis Superior Satyrs Subfamily Satyrinae National Forest £ Common Wood-nymph Cercyonis pegala £ Common Ringlet Coenonympha tullia £ Northern Pearly-eye Enodia anthedon Skipperlings Subfamily Heteropterinae £ Arctic Skipper Carterocephalus palaemon £ Mancinus Alpine Erebia disa mancinus R9SS £ Red-disked Alpine Erebia discoidalis R9SS £ Little Wood-satyr Megisto cymela Grass-Skippers Subfamily Hesperiinae £ Pepper & Salt Skipper Amblyscirtes hegon £ Macoun’s Arctic Oeneis macounii £ Common Roadside-Skipper Amblyscirtes vialis £ Jutta Arctic Oeneis jutta (R9SS) £ Least Skipper Ancyloxypha numitor Northern Crescent £ Eyed Brown Satyrodes eurydice £ Dun Skipper Euphyes vestris Phyciodes selenis £ Common Branded Skipper Hesperia comma £ Indian Skipper Hesperia sassacus Monarchs Subfamily Danainae £ Hobomok Skipper Poanes hobomok £ Monarch Danaus plexippus £ Long Dash Polites mystic £ Peck’s Skipper Polites peckius £ Tawny-edged Skipper Polites themistocles £ European Skipper Thymelicus lineola LINKS: http://www.naba.org/ The U.S. Department of Agriculture (USDA) prohibits discrimination http://www.butterfliesandmoths.org/ in all its programs and activities on the basis of race, color, national -

SOME NOTES on BOLORIA in CENTRAL COLORADO (NYMPHALIDJE) by F. MARTIN BROWN Six Species of Boloria Are Found in Colorado, And
64 Val.S: nos.3-4 SOME NOTES ON BOLORIA IN CENTRAL COLORADO (NYMPHALIDJE) by F. MARTIN BROWN Six species of Boloria are found in Colorado, and there is the possibility that three others fly in the state but as yet have not been discovered. To avoid nomenclatorial confusion while awaiting KLOTS' promised revision of the genus I use here the names for species accepted by McDUNNOUGH in his J 938 Checklist. Boloria myrina ( = selene) tollandensis Barnes & Benjamin My experience with this insect is limited to three areas and all-too-brief collecting. The butterfly is on the wing early in July and by the last week of the month is in rather shabby condition. It seems to prefer open grassy meadows much like those in which its eastern counterpart flies. Altitudinally it seems to be narrowly restricted to a few hundred feet, either way, from 9000 feet. I know of colonies in the Front Range north of the South Fork of the Platte River, the Park Range, the Collegiate Range, and the Rabbit ears Range. DISTRIBUTION OF COLORADO WILLOW-BOG BOLOR/A. Boloria apbirape alticola Barnes & McDunnough This is one of three species closely associated with willow bogs in Colorado. There is good reason to believe that it flies in all of the mountain ranges of the state, although I have nor seen specimens from the Sange do Cristos. It first appears during the last week of June in bogs around 9600 feet, and the last specimens to be taken are found at about 12,000 feet late in August. -

Quaderni Del Museo Civico Di Storia Naturale Di Ferrara
ISSN 2283-6918 Quaderni del Museo Civico di Storia Naturale di Ferrara Anno 2018 • Volume 6 Q 6 Quaderni del Museo Civico di Storia Naturale di Ferrara Periodico annuale ISSN. 2283-6918 Editor: STEFA N O MAZZOTT I Associate Editors: CARLA CORAZZA , EM A N UELA CAR I A ni , EN R ic O TREV is A ni Museo Civico di Storia Naturale di Ferrara, Italia Comitato scientifico / Advisory board CE S ARE AN DREA PA P AZZO ni FI L ipp O Picc OL I Università di Modena Università di Ferrara CO S TA N ZA BO N AD im A N MAURO PELL I ZZAR I Università di Ferrara Ferrara ALE ss A N DRO Min ELL I LU ci O BO N ATO Università di Padova Università di Padova MAURO FA S OLA Mic HELE Mis TR I Università di Pavia Università di Ferrara CARLO FERRAR I VALER I A LE nci O ni Università di Bologna Museo delle Scienze di Trento PI ETRO BRA N D M AYR CORRADO BATT is T I Università della Calabria Università Roma Tre MAR C O BOLOG N A Nic KLA S JA nss O N Università di Roma Tre Linköping University, Sweden IRE N EO FERRAR I Università di Parma In copertina: Fusto fiorale di tornasole comune (Chrozophora tintoria), foto di Nicola Merloni; sezione sottile di Micrite a foraminiferi planctonici del Cretacico superiore (Maastrichtiano), foto di Enrico Trevisani; fiore di digitale purpurea (Digitalis purpurea), foto di Paolo Cortesi; cardo dei lanaioli (Dipsacus fullonum), foto di Paolo Cortesi; ala di macaone (Papilio machaon), foto di Paolo Cortesi; geco comune o tarantola (Tarentola mauritanica), foto di Maurizio Bonora; occhio della sfinge del gallio (Macroglossum stellatarum), foto di Nicola Merloni; bruco della farfalla Calliteara pudibonda, foto di Maurizio Bonora; piumaggio di pernice dei bambù cinese (Bambusicola toracica), foto dell’archivio del Museo Civico di Lentate sul Seveso (Monza). -

Breeding Biology of Blue-Eared Kingfisher Alcedo Meninting Sachin Balkrishna Palkar
PALKAR: Blue-eared Kingfisher 85 Breeding biology of Blue-eared Kingfisher Alcedo meninting Sachin Balkrishna Palkar Palkar, S. B., 2016. Breeding biology of Blue-eared Kingfisher Alcedo meninting. Indian BIRDS 11 (4): 85–90. Sachin Balkrishna Palkar, Near D. B. J. College Gymkhana, Sathyabhama Sadan, House No. 100, Mumbai–Goa highway, Chiplun 415605, Ratnagiri District, Maharashtra, India. E-mail: [email protected] Manuscript received on 30 November 2015. Abstract The breeding biology of the Blue-eared Kingfisher Alcedo meninting was studied in Ratnagiri District, Maharashtra, India, between 2012 and 2015. Thirteen clutches of four pairs were studied. Its breeding season extended from June till September. Pairs excavated tunnels ranging in lengths from 18 to 30 cm, with nest entrance diameters varying from 5.3 to 6.0 cm. The same pair probably reuse a nest across years. A typical clutch comprised six eggs. The incubation period was 21 days (20–23 days), while fledgling period was 23 days (20–27 days). Almost 40% of the nests were double-brooded, which ratio probably depends on the strength of the monsoon. Of 75 eggs laid, 66 hatched (88%), of which 60 fledged (90.9%; a remarkable breeding success of 80%. Introduction and not phillipsi. It is also found in the Andaman Islands (A. The Blue-eared KingfisherAlcedo meninting [113, 114] is m. rufiagastra), where it is, apparently, more abundant than morphologically similar to the Common KingfisherA. atthis but the Common Kingfisher, contrary to its status elsewhere in its is neither as common, nor as widely distributed, in India, as the range (Rasmussen & Anderton 2012).