Sympatric Speciation in Palms on an Oceanic Island
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Howea Forsteriana: Kentia Palm1 Samar Shawaqfeh and Timothy Broschat2
ENH456 Howea forsteriana: Kentia Palm1 Samar Shawaqfeh and Timothy Broschat2 The kentia palm, also known as the sentry palm, is native to Lord Howe Island off the east coast of Australia. It is a slow growing palm that can reach 40 feet in height with a spread of 6–10 feet (Figure 1). It has single slender trunk, 5–6 inches in diameter, that is dark green when young but turns brown as it ages and is exposed to sun. The trunk is attractively ringed with the scars of shed fronds. Leaves are pinnate, or feather-shaped, about 7 ft long, with unarmed petioles 3–4 feet in length. The kentia palm is considered one of the best interior palms for its durability and elegant appearance (Figure 2). The dark green graceful crown of up to three dozen leaves gives it a tropical appearance. Con- tainerized palms can be used on a deck or patio in a shady location or the palm can be planted into the landscape. Kentia palms prefer shade to partial shade but still can adapt to full sun if planted outside. This species can tolerate temperatures of 100°F if not in direct sun. Kentia palms prefer coastal southern California rather than areas like southern Florida or Hawaii because high temperatures, humidity, and rainfall are poorly tolerated. Kentia palms Figure 1. Mature kentia palm in the landscape. Credits: T. K. Broschat are considered to be moderately tolerant of salt spray and can tolerate cold down to 25°F, making them suitable for Kentia palm requires some sun exposure to produce its growing in USDA plant hardiness zones 9b (25–30°F) to creamy flowers. -

Are Introduced Rats (Rattus Rattus) Both Seed Predators and Dispersers In
CHAPTER FOUR: ARE INTRODUCED RATS (RATTUS RATTUS) BOTH SEED PREDATORS AND DISPERSERS IN HAWAII? Aaron B. Shiels Department of Botany University of Hawaii at Manoa 3190 Maile Way Honolulu, HI. 96822 122 Abstract Invasive rodents are among the most ubiquitous and problematic species introduced to islands; more than 80% of the world‘s island groups have been invaded. Introduced rats (black rat, Rattus rattus; Norway rat, R. norvegicus; Pacific rat, R. exulans) are well known as seed predators but are often overlooked as potential seed dispersers despite their common habit of transporting fruits and seeds prior to consumption. The relative likelihood of seed predation and dispersal by the black rat, which is the most common rat in Hawaiian forest, was tested with field and laboratory experiments. In the field, fruits of eight native and four non-native common woody plant species were arranged individually on the forest floor in four treatments that excluded vertebrates of different sizes. Eleven species had a portion (3% to 100%) of their fruits removed from vertebrate-accessible treatments, and automated cameras photographed only black rats removing fruit. In the laboratory, black rats were offered fruits of all 12 species to assess consumption and seed fate. Seeds of two species (non-native Clidemia hirta and native Kadua affinis) passed intact through the digestive tracts of rats. Most of the remaining larger-seeded species had their seeds chewed and destroyed, but for several of these, some partly damaged or undamaged seeds survived rat exposure. The combined field and laboratory findings indicate that many interactions between black rats and seeds of native and non-native plants may result in dispersal. -

Will Climate Change, Genetic and Demographic Variation Or Rat Predation Pose the Greatest Risk for Persistence of an Altitudinally Distributed Island Endemic?
Biology 2012, 1, 736-765; doi:10.3390/biology1030736 OPEN ACCESS biology ISSN 2079-7737 www.mdpi.com/journal/biology Article Will Climate Change, Genetic and Demographic Variation or Rat Predation Pose the Greatest Risk for Persistence of an Altitudinally Distributed Island Endemic? Catherine Laura Simmons 1, Tony D. Auld 2, Ian Hutton 3, William J. Baker 4 and Alison Shapcott 1,* 1 Faculty of Science Health, Education and Engineering, University of the Sunshine Coast, Maroochydore DC, QLD 4558, Australia; E-Mail: [email protected] 2 Office of Environment and Heritage (NSW), P.O. Box 1967 Hurstville, NSW 2220, Australia; E-Mail: [email protected] 3 P.O. Box 157, Lord Howe Island, NSW 2898, Australia; E-Mail: [email protected] 4 Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3AB, UK; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +61-7-5430-1211; Fax: +61-7-5430-2881. Received: 3 September 2012; in revised form: 29 October 2012 / Accepted: 16 November 2012 / Published: 23 November 2012 Abstract: Species endemic to mountains on oceanic islands are subject to a number of existing threats (in particular, invasive species) along with the impacts of a rapidly changing climate. The Lord Howe Island endemic palm Hedyscepe canterburyana is restricted to two mountains above 300 m altitude. Predation by the introduced Black Rat (Rattus rattus) is known to significantly reduce seedling recruitment. We examined the variation in Hedyscepe in terms of genetic variation, morphology, reproductive output and demographic structure, across an altitudinal gradient. -
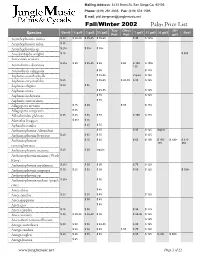
Winter-Fall Sale 2002 Palm Trees-Web
Mailing Address: 3233 Brant St. San Diego Ca, 92103 Phone: (619) 291 4605 Fax: (619) 574 1595 E mail: [email protected] Fall/Winter 2002 Palm Price List Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Acanthophoenix crinita $ 30 $ 30-40 $ 35-45 $ 55-65 $ 95 $ 125+ Acanthophoenix rubra $ 35 Acanthophoenix sp. $ 25+ $ 35+ $ 55+ Acoelorrhaphe wrightii $ 15 $ 300 Acrocomia aculeata $ 25+ $ 35 $ 35-45 $ 65 $ 65 $ 100- $ 150+ Actinokentia divaricata 135 Actinorhytis calapparia $ 55 $ 125 Aiphanes acanthophylla $ 45-55 inquire $ 125 Aiphanes caryotaefolia $ 25 $ 55-65 $ 45-55 $ 85 $ 125 Aiphanes elegans $ 20 $ 35 Aiphanes erosa $ 45-55 $ 125 Aiphanes lindeniana $ 55 $ 125 Aiphanes vincentsiana $ 55 Allagoptera arenaria $ 25 $ 40 $ 55 $ 135 Allagoptera campestris $ 35 Alloschmidtia glabrata $ 35 $ 45 $ 55 $ 85 $ 150 $ 175 Alsmithia longipes $ 35+ $ 55 Aphandra natalia $ 35 $ 55 Archontophoenix Alexandrae $ 55 $ 85 $ 125 inquire Archontophoenix Beatricae $ 20 $ 35 $ 55 $ 125 Archontophoenix $ 25 $ 45 $ 65 $ 100 $ 150- $ 200+ $ 310- 175 350 cunninghamiana Archontophoenix maxima $ 25 $ 30 inquire Archontophoenix maxima (Wash River) Archontophoenix myolaensis $ 25+ $ 30 $ 50 $ 75 $ 125 Archontophoenix purpurea $ 30 $ 25 $ 35 $ 50 $ 85 $ 125 $ 300+ Archontophoenix sp. Archontophoenix tuckerii (peach $ 25+ $ 55 river) Areca alicae $ 45 Areca catechu $ 20 $ 35 $ 45 $ 125 Areca guppyana $ 30 $ 45 Areca ipot $ 45 Areca triandra $ 25 $ 30 $ 95 $ 125 Areca vestiaria $ 25 $ 30-35 $ 35-40 $ 55 $ 85-95 $ 125 Arecastrum romanzoffianum $ 125 Arenga australasica $ 20 $ 30 $ 35 $ 45-55 $ 85 $ 125 Arenga caudata $ 20 $ 30 $ 45 $ 55 $ 75 $ 100 Arenga engleri $ 20 $ 60 $ 35 $ 45 $ 85 $ 125 $ 200 $ 300+ Arenga hastata $ 25 www.junglemusic.net Page 1 of 22 Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Arenga hookeriana inquire Arenga micranthe 'Lhutan' $ 20 inquire Arenga pinnata $ 35 $ 50 $ 85 $ 125 Arenga sp. -

Saving Tahina — You Can Help
July 2020 NEWSLETTER SAVING TAHINA — YOU CAN HELP PalmTalk (the public forum of the International Palm Society) is initiating a pro- gram to preserve Tahina spectabilis, an endangered palm that PalmTalk brought to the world’s attention in 2007. The discovery of this enormous, new palm gen- erated worldwide interest, but now due to its extremely limited and fragile range in Madagascar, Tahina needs our help. Please visit PalmTalk (go to www. palms.org, and click on the PalmTalk Forums tab) to see how the IPS and the Roy- al Botanic Gardens, Kew are partnering with a local village in Madagascar to help save this amazing palm. You can make a donation to the pro- ject and be a part of this unique partnership in conser- vation. Please donate today! One of the first photos posted on PalmTalk of the mysterious palm that came to be known as Tahina spectabilis and the little girl (at the time) for whom it was named, Anne-Tahina Metz. Volume 8.06 · July 2020 · Newsletter of the International Palm Society | Editor: Andy Hurwitz [email protected] Virtual hiking on Reunion Island, lowlands editionby Andy Hurwitz This article continues the “virtual hike” and tour of Reunion’s palms that began in last month’s News- letter. This month, we explore the palms and landscapes of the low to middle elevations. We begin by traveling to the windward eastern coast of the island, just south of Piton-Sainte-Rose, to ramble in Anse des Cascades. This idyllic cove is situated among groves of palm trees. The park is cool and shady. -

2951 – 3000 Trinidad to British Guiana
2951 – 3000 Trinidad to British Guiana [Inside cover] Book 9 Acanthophoenix 2956 Lecythis 2963 Acrocomia 2961 Licuala 2978 Ananas 2993 Livistona 2982 Archontophoenix 2983 Lodoicea 2985 Areca 2953 2953 Mauritia 2984 “ 2954 Mokka-mokka 2997 Astrocaryum 2957 Nipa 2981 “ 2986 Pachira 2976 “ 2987 “ 3000 Asystasia 2964 Passiflora 2952 Bauhinia 2960 “ 2995 Borassus 2979 Peltogyne 2970 Bromelia 2996 Pithecolobium 2965 Caryocar 2999 Randia 2994 Citrus 2998 Samanea 2966 Copernicia 2977 Undetermined 2967 Crotolaria 2973 “ In ink:]Ochna 2971 “ 2974 “ 2972 Desmoncus 2951 “ 2989 Diospyrus 2968 “ 2990 Euterpe 2955 “ 2991 Gmelina 2969 “ 2992 Hyphaene 2980 Zingerberaciae 2958 Ixora 2975 TILLANDSIA 2996 Jacaranda 2962 2951 Demoncus minor? See Harold Loomis’ notes and photo of inflorescence. #54 Villainously spacing & hooked climbing palm reminding one of those terrible Rattan palms of the Orient. When in fruit its bunches of deep scarlet fruits are attractive. A denizen of the deep shade forest and requiring moisture. From my experience in Coconut Grove with this genus I judge it wants half shade. Collected in Arena Forest Reserve Trinidad. 2/4/32 [In pencil] Loomis Photo 220 See D. F. Photo 18454-5 2952 P. H. Dorsett [In ink] “rubra?” Passiflora Sp Wild species of passion vine collected in the outskirts of the town of Eleuthera Bluff Island of Eleuthera Bahamas. Attractive looking species useful [Break in text, in ink] 1 single plant in pot Dec. 14/32. C. F. [Continuation of text] for breeding purposes. 1-7-32 (I’d like a few seeds or plants for my passiflora collection in Coconut Grove) Passiflora sp. Wild species growing in pothole in rocky soil of Eleuthera Bluff a colored town on Eleuthera Island. -

Bio 308-Course Guide
COURSE GUIDE BIO 308 BIOGEOGRAPHY Course Team Dr. Kelechi L. Njoku (Course Developer/Writer) Professor A. Adebanjo (Programme Leader)- NOUN Abiodun E. Adams (Course Coordinator)-NOUN NATIONAL OPEN UNIVERSITY OF NIGERIA BIO 308 COURSE GUIDE National Open University of Nigeria Headquarters 14/16 Ahmadu Bello Way Victoria Island Lagos Abuja Office No. 5 Dar es Salaam Street Off Aminu Kano Crescent Wuse II, Abuja e-mail: [email protected] URL: www.nou.edu.ng Published by National Open University of Nigeria Printed 2013 ISBN: 978-058-434-X All Rights Reserved Printed by: ii BIO 308 COURSE GUIDE CONTENTS PAGE Introduction ……………………………………......................... iv What you will Learn from this Course …………………............ iv Course Aims ……………………………………………............ iv Course Objectives …………………………………………....... iv Working through this Course …………………………….......... v Course Materials ………………………………………….......... v Study Units ………………………………………………......... v Textbooks and References ………………………………........... vi Assessment ……………………………………………….......... vi End of Course Examination and Grading..................................... vi Course Marking Scheme................................................................ vii Presentation Schedule.................................................................... vii Tutor-Marked Assignment ……………………………….......... vii Tutors and Tutorials....................................................................... viii iii BIO 308 COURSE GUIDE INTRODUCTION BIO 308: Biogeography is a one-semester, 2 credit- hour course in Biology. It is a 300 level, second semester undergraduate course offered to students admitted in the School of Science and Technology, School of Education who are offering Biology or related programmes. The course guide tells you briefly what the course is all about, what course materials you will be using and how you can work your way through these materials. It gives you some guidance on your Tutor- Marked Assignments. There are Self-Assessment Exercises within the body of a unit and/or at the end of each unit. -

Lepidorrhachis Mooreana (H
Palm Conservation – Palm Specialist Group Lepidorrhachis mooreana (H. Wendl. & Drude) O. F. Cook Status: Not Evaluated in IUCN Red List. Vulnerable according to Dowe in Johnson (1996). Preliminary evaluation based on IUCN 2001 criteria: Endangered (EN B1a,bv) Common name Little Mountain Palm. Natural range Lepidorrhachis mooreana is restricted to the summits of Mt. Gower (875 m) and Mt. Lidgbird (777 m) on the remote Lord Howe Island. It occurs only above 750 m in dwarf mossy forest that dominates the summit plateau of Mt. Gower and the narrow summit ridge of Mt. Lidgbird. This forest is home to numerous remarkable endemic species including the pumpkin tree (Negria rhabdothamnoides), an arborescent member of the Gesneriaceae, and Dracophyllum fitzgeraldii (Ericaceae). It is also the primary nesting locality of the providence petrel (Pterodroma solandri) and is a stronghold for the woodhen (Tricholimnas sylvestris), an endemic member of the rail family that was recently rescued from the brink of extinction. However, less that 0.5 km2 of Lord Howe’s total surface area of 12 km2 is found above 750 m. The total area of suitable habitat available to Lepidorrhachis is thus extremely limited. Recognition characteristics Lepidorrhachis is very easily distinguished from the two other endemic palm genera on Lord Howe Island, Howea and Hedyscepe. It is a short solitary palm with a stem that rarely exceeds 2 m in height. It has stiff, arching leaves with short, deeply split leaf sheaths that do not form a distinct crownshaft. The sheaths are also covered with buff indumentum. Its bushy inflorescences are born below the leaves and are unisexual, both male and female inflorescences occurring on the same plant. -

The Island Rule and Its Application to Multiple Plant Traits
The island rule and its application to multiple plant traits Annemieke Lona Hedi Hendriks A thesis submitted to the Victoria University of Wellington in partial fulfilment of the requirements for the degree of Master of Science in Ecology and Biodiversity Victoria University of Wellington, New Zealand 2019 ii “The larger the island of knowledge, the longer the shoreline of wonder” Ralph W. Sockman. iii iv General Abstract Aim The Island Rule refers to a continuum of body size changes where large mainland species evolve to become smaller and small species evolve to become larger on islands. Previous work focuses almost solely on animals, with virtually no previous tests of its predictions on plants. I tested for (1) reduced floral size diversity on islands, a logical corollary of the island rule and (2) evidence of the Island Rule in plant stature, leaf size and petiole length. Location Small islands surrounding New Zealand; Antipodes, Auckland, Bounty, Campbell, Chatham, Kermadec, Lord Howe, Macquarie, Norfolk, Snares, Stewart and the Three Kings. Methods I compared the morphology of 65 island endemics and their closest ‘mainland’ relative. Species pairs were identified. Differences between archipelagos located at various latitudes were also assessed. Results Floral sizes were reduced on islands relative to the ‘mainland’, consistent with predictions of the Island Rule. Plant stature, leaf size and petiole length conformed to the Island Rule, with smaller plants increasing in size, and larger plants decreasing in size. Main conclusions Results indicate that the conceptual umbrella of the Island Rule can be expanded to plants, accelerating understanding of how plant traits evolve on isolated islands. -

Wendland's Palms
Wendland’s Palms Hermann Wendland (1825 – 1903) of Herrenhausen Gardens, Hannover: his contribution to the taxonomy and horticulture of the palms ( Arecaceae ) John Leslie Dowe Published by the Botanic Garden and Botanical Museum Berlin as Englera 36 Serial publication of the Botanic Garden and Botanical Museum Berlin November 2019 Englera is an international monographic series published at irregular intervals by the Botanic Garden and Botanical Museum Berlin (BGBM), Freie Universität Berlin. The scope of Englera is original peer-reviewed material from the entire fields of plant, algal and fungal taxonomy and systematics, also covering related fields such as floristics, plant geography and history of botany, provided that it is monographic in approach and of considerable volume. Editor: Nicholas J. Turland Production Editor: Michael Rodewald Printing and bookbinding: Laserline Druckzentrum Berlin KG Englera online access: Previous volumes at least three years old are available through JSTOR: https://www.jstor.org/journal/englera Englera homepage: https://www.bgbm.org/englera Submission of manuscripts: Before submitting a manuscript please contact Nicholas J. Turland, Editor of Englera, Botanic Garden and Botanical Museum Berlin, Freie Universität Berlin, Königin- Luise-Str. 6 – 8, 14195 Berlin, Germany; e-mail: [email protected] Subscription: Verlagsauslieferung Soyka, Goerzallee 299, 14167 Berlin, Germany; e-mail: kontakt@ soyka-berlin.de; https://shop.soyka-berlin.de/bgbm-press Exchange: BGBM Press, Botanic Garden and Botanical Museum Berlin, Freie Universität Berlin, Königin-Luise-Str. 6 – 8, 14195 Berlin, Germany; e-mail: [email protected] © 2019 Botanic Garden and Botanical Museum Berlin, Freie Universität Berlin All rights (including translations into other languages) reserved. -

Plant and Landscape Guide Rancho Santa Fe, California, Is Considered to Be in a Very High Fire Hazard Severity Zone Because of Its Unique Characteristics
Plant and Landscape Guide Rancho Santa Fe, California, is considered to be in a very high fire hazard severity zone because of its unique characteristics. It is considered a Wildland Urban Interface area because of the proximity of the natural chaparral vegetation to developed areas, often immediately abutting structures. Additionally, warm coastal weather, Santa Ana winds, mountainous terrain, and steep slopes contribute to the very high fire hazard severity zone designation. DistrictIn an effort (RSFFPD) to protect does homes not allow from certain a future types devastating of trees, Wildlandplants, or fire shrubs such to as be the ones experienced in 2003 and 2007, the Rancho Santa Fe Fire Protection planted within certain distances of structures. This booklet contains valuable educateinformation the publicpertaining on RSFFPD’s to both desirable ordinances and regarding undesirable landscaping trees, shrubs, so they can ground covers, vines, roadway clearances, and palm trees. The goal is to Lady Bank’s Rose increase the the chances of their home surviving a wildfire. Please feel free to contactPlease Note: the Fire District if you have any questions, comments, or concerns. 1. THIS IS NOT A COMPREHENSIVE LIST. This booklet is intended to simply guide the public on what types of trees and shrubs are acceptable within the Fire District. Other trees and shrubs not listed 2. may also be acceptable upon approval by the RSFFPD. Trees listed as requiring 30-foot spacing from the drip line to the structure are considered non-fire resistive trees by the RSFFPD. Consult a design professional or the Fire District for site-specific 3. -

1 Ornamental Palms
1 Ornamental Palms: Biology and Horticulture T.K. Broschat and M.L. Elliott Fort Lauderdale Research and Education Center University of Florida, Davie, FL 33314, USA D.R. Hodel University of California Cooperative Extension Alhambra, CA 91801, USA ABSTRACT Ornamental palms are important components of tropical, subtropical, and even warm temperate climate landscapes. In colder climates, they are important interiorscape plants and are often a focal point in malls, businesses, and other public areas. As arborescent monocots, palms have a unique morphology and this greatly influences their cultural requirements. Ornamental palms are over- whelmingly seed propagated, with seeds of most species germinating slowly and being intolerant of prolonged storage or cold temperatures. They generally do not have dormancy requirements, but do require high temperatures (30–35°C) for optimum germination. Palms are usually grown in containers prior to trans- planting into a field nursery or landscape. Because of their adventitious root system, large field-grown specimen palms can easily be transplanted. In the landscape, palm health and quality are greatly affected by nutritional deficien- cies, which can reduce their aesthetic value, growth rate, or even cause death. Palm life canCOPYRIGHTED also be shortened by a number of MATERIAL diseases or insect pests, some of which are lethal, have no controls, or have wide host ranges. With the increasing use of palms in the landscape, pathogens and insect pests have moved with the Horticultural Reviews, Volume 42, First Edition. Edited by Jules Janick. 2014 Wiley-Blackwell. Published 2014 by John Wiley & Sons, Inc. 1 2 T.K. BROSCHAT, D.R. HODEL, AND M.L.