(Hemiptera: Heteroptera: Hydrocorisae)* (1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insetos Do Brasil
COSTA LIMA INSETOS DO BRASIL 2.º TOMO HEMÍPTEROS ESCOLA NACIONAL DE AGRONOMIA SÉRIE DIDÁTICA N.º 3 - 1940 INSETOS DO BRASIL 2.º TOMO HEMÍPTEROS A. DA COSTA LIMA Professor Catedrático de Entomologia Agrícola da Escola Nacional de Agronomia Ex-Chefe de Laboratório do Instituto Oswaldo Cruz INSETOS DO BRASIL 2.º TOMO CAPÍTULO XXII HEMÍPTEROS ESCOLA NACIONAL DE AGRONOMIA SÉRIE DIDÁTICA N.º 3 - 1940 CONTEUDO CAPÍTULO XXII PÁGINA Ordem HEMÍPTERA ................................................................................................................................................ 3 Superfamília SCUTELLEROIDEA ............................................................................................................ 42 Superfamília COREOIDEA ............................................................................................................................... 79 Super família LYGAEOIDEA ................................................................................................................................. 97 Superfamília THAUMASTOTHERIOIDEA ............................................................................................... 124 Superfamília ARADOIDEA ................................................................................................................................... 125 Superfamília TINGITOIDEA .................................................................................................................................... 132 Superfamília REDUVIOIDEA ........................................................................................................................... -

Torix Rickettsia Are Widespread in Arthropods and Reflect a Neglected Symbiosis
GigaScience, 10, 2021, 1–19 doi: 10.1093/gigascience/giab021 RESEARCH RESEARCH Torix Rickettsia are widespread in arthropods and Downloaded from https://academic.oup.com/gigascience/article/10/3/giab021/6187866 by guest on 05 August 2021 reflect a neglected symbiosis Jack Pilgrim 1,*, Panupong Thongprem 1, Helen R. Davison 1, Stefanos Siozios 1, Matthew Baylis1,2, Evgeny V. Zakharov3, Sujeevan Ratnasingham 3, Jeremy R. deWaard3, Craig R. Macadam4,M. Alex Smith5 and Gregory D. D. Hurst 1 1Institute of Infection, Veterinary and Ecological Sciences, Faculty of Health and Life Sciences, University of Liverpool, Leahurst Campus, Chester High Road, Neston, Wirral CH64 7TE, UK; 2Health Protection Research Unit in Emerging and Zoonotic Infections, University of Liverpool, 8 West Derby Street, Liverpool L69 7BE, UK; 3Centre for Biodiversity Genomics, University of Guelph, 50 Stone Road East, Guelph, Ontario N1G2W1, Canada; 4Buglife – The Invertebrate Conservation Trust, Balallan House, 24 Allan Park, Stirling FK8 2QG, UK and 5Department of Integrative Biology, University of Guelph, Summerlee Science Complex, Guelph, Ontario N1G 2W1, Canada ∗Correspondence address. Jack Pilgrim, Institute of Infection, Veterinary and Ecological Sciences, Faculty of Health and Life Sciences, University of Liverpool, Liverpool, UK. E-mail: [email protected] http://orcid.org/0000-0002-2941-1482 Abstract Background: Rickettsia are intracellular bacteria best known as the causative agents of human and animal diseases. Although these medically important Rickettsia are often transmitted via haematophagous arthropods, other Rickettsia, such as those in the Torix group, appear to reside exclusively in invertebrates and protists with no secondary vertebrate host. Importantly, little is known about the diversity or host range of Torix group Rickettsia. -

Biological Control of Insect Pests in the Tropics - M
TROPICAL BIOLOGY AND CONSERVATION MANAGEMENT – Vol. III - Biological Control of Insect Pests In The Tropics - M. V. Sampaio, V. H. P. Bueno, L. C. P. Silveira and A. M. Auad BIOLOGICAL CONTROL OF INSECT PESTS IN THE TROPICS M. V. Sampaio Instituto de Ciências Agrária, Universidade Federal de Uberlândia, Brazil V. H. P. Bueno and L. C. P. Silveira Departamento de Entomologia, Universidade Federal de Lavras, Brazil A. M. Auad Embrapa Gado de Leite, Empresa Brasileira de Pesquisa Agropecuária, Brazil Keywords: Augmentative biological control, bacteria, classical biological control, conservation of natural enemies, fungi, insect, mite, natural enemy, nematode, predator, parasitoid, pathogen, virus. Contents 1. Introduction 2. Natural enemies of insects and mites 2.1. Entomophagous 2.1.1. Predators 2.1.2. Parasitoids 2.2. Entomopathogens 2.2.1. Fungi 2.2.2. Bacteria 2.2.3. Viruses 2.2.4. Nematodes 3. Categories of biological control 3.1. Natural Biological Control 3.2. Applied Biological Control 3.2.1. Classical Biological Control 3.2.2. Augmentative Biological Control 3.2.3. Conservation of Natural Enemies 4. Conclusions Glossary UNESCO – EOLSS Bibliography Biographical Sketches Summary SAMPLE CHAPTERS Biological control is a pest control method with low environmental impact and small contamination risk for humans, domestic animals and the environment. Several success cases of biological control can be found in the tropics around the world. The classical biological control has been applied with greater emphasis in Australia and Latin America, with many success cases of exotic natural enemies’ introduction for the control of exotic pests. Augmentative biocontrol is used in extensive areas in Latin America, especially in the cultures of sugar cane, coffee, and soybeans. -

Diversity of Water Bugs in Gujranwala District, Punjab, Pakistan
Journal of Bioresource Management Volume 5 Issue 1 Article 1 Diversity of Water Bugs in Gujranwala District, Punjab, Pakistan Muhammad Shahbaz Chattha Women University Azad Jammu & Kashmir, Bagh (AJK), [email protected] Abu Ul Hassan Faiz Women University of Azad Jammu & Kashmir, Bagh (AJK), [email protected] Arshad Javid University of Veterinary & Animal Sciences, Lahore, [email protected] Irfan Baboo Cholistan University of Veterinary & Animal Sciences, Bahawalpur, [email protected] Inayat Ullah Malik The University of Lakki Marwat, Lakki Marwat, [email protected] Follow this and additional works at: https://corescholar.libraries.wright.edu/jbm Part of the Aquaculture and Fisheries Commons, Biodiversity Commons, Entomology Commons, Terrestrial and Aquatic Ecology Commons, and the Zoology Commons Recommended Citation Chattha, M. S., Faiz, A. H., Javid, A., Baboo, I., & Malik, I. U. (2018). Diversity of Water Bugs in Gujranwala District, Punjab, Pakistan, Journal of Bioresource Management, 5 (1). DOI: https://doi.org/10.35691/JBM.8102.0081 ISSN: 2309-3854 online (Received: May 16, 2019; Accepted: Sep 19, 2019; Published: Jan 1, 2018) This Article is brought to you for free and open access by CORE Scholar. It has been accepted for inclusion in Journal of Bioresource Management by an authorized editor of CORE Scholar. For more information, please contact [email protected]. Diversity of Water Bugs in Gujranwala District, Punjab, Pakistan © Copyrights of all the papers published in Journal of Bioresource Management are with its publisher, Center for Bioresource Research (CBR) Islamabad, Pakistan. This permits anyone to copy, redistribute, remix, transmit and adapt the work for non-commercial purposes provided the original work and source is appropriately cited. -

UFRJ a Paleoentomofauna Brasileira
Anuário do Instituto de Geociências - UFRJ www.anuario.igeo.ufrj.br A Paleoentomofauna Brasileira: Cenário Atual The Brazilian Fossil Insects: Current Scenario Dionizio Angelo de Moura-Júnior; Sandro Marcelo Scheler & Antonio Carlos Sequeira Fernandes Universidade Federal do Rio de Janeiro, Programa de Pós-Graduação em Geociências: Patrimônio Geopaleontológico, Museu Nacional, Quinta da Boa Vista s/nº, São Cristóvão, 20940-040. Rio de Janeiro, RJ, Brasil. E-mails: [email protected]; [email protected]; [email protected] Recebido em: 24/01/2018 Aprovado em: 08/03/2018 DOI: http://dx.doi.org/10.11137/2018_1_142_166 Resumo O presente trabalho fornece um panorama geral sobre o conhecimento da paleoentomologia brasileira até o presente, abordando insetos do Paleozoico, Mesozoico e Cenozoico, incluindo a atualização das espécies publicadas até o momento após a última grande revisão bibliográica, mencionando ainda as unidades geológicas em que ocorrem e os trabalhos relacionados. Palavras-chave: Paleoentomologia; insetos fósseis; Brasil Abstract This paper provides an overview of the Brazilian palaeoentomology, about insects Paleozoic, Mesozoic and Cenozoic, including the review of the published species at the present. It was analiyzed the geological units of occurrence and the related literature. Keywords: Palaeoentomology; fossil insects; Brazil Anuário do Instituto de Geociências - UFRJ 142 ISSN 0101-9759 e-ISSN 1982-3908 - Vol. 41 - 1 / 2018 p. 142-166 A Paleoentomofauna Brasileira: Cenário Atual Dionizio Angelo de Moura-Júnior; Sandro Marcelo Schefler & Antonio Carlos Sequeira Fernandes 1 Introdução Devoniano Superior (Engel & Grimaldi, 2004). Os insetos são um dos primeiros organismos Algumas ordens como Blattodea, Hemiptera, Odonata, Ephemeroptera e Psocopera surgiram a colonizar os ambientes terrestres e aquáticos no Carbonífero com ocorrências até o recente, continentais (Engel & Grimaldi, 2004). -

The Corixidae (Hemiptera) of Oklahoma KURT F
BIOLOGICAL SCIENCES 71 The Corixidae (Hemiptera) of Oklahoma KURT F. SCHAEFER, Panhandle State Colle.e, Goodwell The Corixidae or water boatman family is a commonly collected fam ily taken in a variety of aquatic habitats and frequently at lights at night or on shiny surfaces during the day. Hungerford's 1948 monograph on the world corixids is an important contribution, essential to a serious collector. My paper is an attempt to make the identification of state fonns easier and to supply descriptions and distribution data for the corixids of the state. Schaefer and Drew (1964) reported 18 species and Ewing (1964) added one for the state. Five addi tional species are included because information of their known ranges in dicates that they will probably be found in Oklahoma when more collecting is done. Each pair of legs is modified for a different function. The anterior pair is short with the tenninal segment (pala) often more or less spoon shaped and fringed with bristles for food gathering. Both adults and nymphs feed mainly on algae and protozoa, obtained from bottom ooze (Usinger, 1956). The middle pair of legs, used for anchorage and support, is long and slender, tenninating with two long claws. The hind pair, for swimming, is stouter, laterally flattened and fringed with hairs. The principal dimorphic structures used as key characters are as fol lows: males, usually smaller, with vertex of the head otten more produced and frons concavely depressed. Fonn and chaetotaxy of the male palae, front tarsi, are much used characters. The female abdomen is bilaterally symmetrical, while the asymmetry of male may be either to the right (dextral) or left (stnistral). -

Metacommunities and Biodiversity Patterns in Mediterranean Temporary Ponds: the Role of Pond Size, Network Connectivity and Dispersal Mode
METACOMMUNITIES AND BIODIVERSITY PATTERNS IN MEDITERRANEAN TEMPORARY PONDS: THE ROLE OF POND SIZE, NETWORK CONNECTIVITY AND DISPERSAL MODE Irene Tornero Pinilla Per citar o enllaçar aquest document: Para citar o enlazar este documento: Use this url to cite or link to this publication: http://www.tdx.cat/handle/10803/670096 http://creativecommons.org/licenses/by-nc/4.0/deed.ca Aquesta obra està subjecta a una llicència Creative Commons Reconeixement- NoComercial Esta obra está bajo una licencia Creative Commons Reconocimiento-NoComercial This work is licensed under a Creative Commons Attribution-NonCommercial licence DOCTORAL THESIS Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode Irene Tornero Pinilla 2020 DOCTORAL THESIS Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode IRENE TORNERO PINILLA 2020 DOCTORAL PROGRAMME IN WATER SCIENCE AND TECHNOLOGY SUPERVISED BY DR DANI BOIX MASAFRET DR STÉPHANIE GASCÓN GARCIA Thesis submitted in fulfilment of the requirements to obtain the Degree of Doctor at the University of Girona Dr Dani Boix Masafret and Dr Stéphanie Gascón Garcia, from the University of Girona, DECLARE: That the thesis entitled Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode submitted by Irene Tornero Pinilla to obtain a doctoral degree has been completed under our supervision. In witness thereof, we hereby sign this document. Dr Dani Boix Masafret Dr Stéphanie Gascón Garcia Girona, 22nd November 2019 A mi familia Caminante, son tus huellas el camino y nada más; Caminante, no hay camino, se hace camino al andar. -

Position Specificity in the Genus Coreomyces (Laboulbeniomycetes, Ascomycota)
VOLUME 1 JUNE 2018 Fungal Systematics and Evolution PAGES 217–228 doi.org/10.3114/fuse.2018.01.09 Position specificity in the genus Coreomyces (Laboulbeniomycetes, Ascomycota) H. Sundberg1*, Å. Kruys2, J. Bergsten3, S. Ekman2 1Systematic Biology, Department of Organismal Biology, Evolutionary Biology Centre, Uppsala University, Uppsala, Sweden 2Museum of Evolution, Uppsala University, Uppsala, Sweden 3Department of Zoology, Swedish Museum of Natural History, Stockholm, Sweden *Corresponding author: [email protected] Key words: Abstract: To study position specificity in the insect-parasitic fungal genus Coreomyces (Laboulbeniaceae, Laboulbeniales), Corixidae we sampled corixid hosts (Corixidae, Heteroptera) in southern Scandinavia. We detected Coreomyces thalli in five different DNA positions on the hosts. Thalli from the various positions grouped in four distinct clusters in the resulting gene trees, distinctly Fungi so in the ITS and LSU of the nuclear ribosomal DNA, less so in the SSU of the nuclear ribosomal DNA and the mitochondrial host-specificity ribosomal DNA. Thalli from the left side of abdomen grouped in a single cluster, and so did thalli from the ventral right side. insect Thalli in the mid-ventral position turned out to be a mix of three clades, while thalli growing dorsally grouped with thalli from phylogeny the left and right abdominal clades. The mid-ventral and dorsal positions were found in male hosts only. The position on the left hemelytron was shared by members from two sister clades. Statistical analyses demonstrate a significant positive correlation between clade and position on the host, but also a weak correlation between host sex and clade membership. These results indicate that sex-of-host specificity may be a non-existent extreme in a continuum, where instead weak preferences for one host sex may turn out to be frequent. -

Table of Contents 2
Southwest Association of Freshwater Invertebrate Taxonomists (SAFIT) List of Freshwater Macroinvertebrate Taxa from California and Adjacent States including Standard Taxonomic Effort Levels 1 March 2011 Austin Brady Richards and D. Christopher Rogers Table of Contents 2 1.0 Introduction 4 1.1 Acknowledgments 5 2.0 Standard Taxonomic Effort 5 2.1 Rules for Developing a Standard Taxonomic Effort Document 5 2.2 Changes from the Previous Version 6 2.3 The SAFIT Standard Taxonomic List 6 3.0 Methods and Materials 7 3.1 Habitat information 7 3.2 Geographic Scope 7 3.3 Abbreviations used in the STE List 8 3.4 Life Stage Terminology 8 4.0 Rare, Threatened and Endangered Species 8 5.0 Literature Cited 9 Appendix I. The SAFIT Standard Taxonomic Effort List 10 Phylum Silicea 11 Phylum Cnidaria 12 Phylum Platyhelminthes 14 Phylum Nemertea 15 Phylum Nemata 16 Phylum Nematomorpha 17 Phylum Entoprocta 18 Phylum Ectoprocta 19 Phylum Mollusca 20 Phylum Annelida 32 Class Hirudinea Class Branchiobdella Class Polychaeta Class Oligochaeta Phylum Arthropoda Subphylum Chelicerata, Subclass Acari 35 Subphylum Crustacea 47 Subphylum Hexapoda Class Collembola 69 Class Insecta Order Ephemeroptera 71 Order Odonata 95 Order Plecoptera 112 Order Hemiptera 126 Order Megaloptera 139 Order Neuroptera 141 Order Trichoptera 143 Order Lepidoptera 165 2 Order Coleoptera 167 Order Diptera 219 3 1.0 Introduction The Southwest Association of Freshwater Invertebrate Taxonomists (SAFIT) is charged through its charter to develop standardized levels for the taxonomic identification of aquatic macroinvertebrates in support of bioassessment. This document defines the standard levels of taxonomic effort (STE) for bioassessment data compatible with the Surface Water Ambient Monitoring Program (SWAMP) bioassessment protocols (Ode, 2007) or similar procedures. -

Insects of Larose Forest (Excluding Lepidoptera and Odonates)
Insects of Larose Forest (Excluding Lepidoptera and Odonates) • Non-native species indicated by an asterisk* • Species in red are new for the region EPHEMEROPTERA Mayflies Baetidae Small Minnow Mayflies Baetidae sp. Small minnow mayfly Caenidae Small Squaregills Caenidae sp. Small squaregill Ephemerellidae Spiny Crawlers Ephemerellidae sp. Spiny crawler Heptageniiidae Flatheaded Mayflies Heptageniidae sp. Flatheaded mayfly Leptophlebiidae Pronggills Leptophlebiidae sp. Pronggill PLECOPTERA Stoneflies Perlodidae Perlodid Stoneflies Perlodid sp. Perlodid stonefly ORTHOPTERA Grasshoppers, Crickets and Katydids Gryllidae Crickets Gryllus pennsylvanicus Field cricket Oecanthus sp. Tree cricket Tettigoniidae Katydids Amblycorypha oblongifolia Angular-winged katydid Conocephalus nigropleurum Black-sided meadow katydid Microcentrum sp. Leaf katydid Scudderia sp. Bush katydid HEMIPTERA True Bugs Acanthosomatidae Parent Bugs Elasmostethus cruciatus Red-crossed stink bug Elasmucha lateralis Parent bug Alydidae Broad-headed Bugs Alydus sp. Broad-headed bug Protenor sp. Broad-headed bug Aphididae Aphids Aphis nerii Oleander aphid* Paraprociphilus tesselatus Woolly alder aphid Cicadidae Cicadas Tibicen sp. Cicada Cicadellidae Leafhoppers Cicadellidae sp. Leafhopper Coelidia olitoria Leafhopper Cuernia striata Leahopper Draeculacephala zeae Leafhopper Graphocephala coccinea Leafhopper Idiodonus kelmcottii Leafhopper Neokolla hieroglyphica Leafhopper 1 Penthimia americana Leafhopper Tylozygus bifidus Leafhopper Cercopidae Spittlebugs Aphrophora cribrata -

New Records of Aquatic Heteroptera (Hemiptera) from the Andean Foothills of the Amazonia (Putumayo, Colombia)
Revista Colombiana de Entomología 40 (2): 230-234 (Julio - Diciembre 2014) New records of aquatic Heteroptera (Hemiptera) from the Andean foothills of the Amazonia (Putumayo, Colombia) Nuevos registros de Heteroptera acuáticos (Hemiptera) del piedemonte Andino de la Amazonia (Putumayo, Colombia) DORA NANCY PADILLA-GIL1 Abstract: Four new records of species of aquatic heteropterous are added to the entomofauna of Colombia and, distributional limits are extended for all species collected. New data is reported for the distribution of 18 species, of which two belong to the infraorder Nepomorpha: Corixidae: Heterocorixa and Notonectidae: Martarega; 16 belong to Gerromorpha: of which 12 belong to the family Gerridae: Metrobates, Tachygerris, Limnogonus (two species), Brachymetra, Neogerris, Potamobates, Trepobates (three species), Telmatometra, Rheumatobates; three belong to the family Veliidae: Rhagovelia, Microvelia (two species), and one to Mesoveliidae: Mesovelia. Key words: Aquatic insects. Corixidae. Notonectidae. Gerridae. Veliidae. Resumen: Se adicionan cuatro nuevos registros de especies de heterópteros acuáticos a la entomofauna de Colombia y se extiende el área de distribución para todas las especies colectadas. Un total de 18 especies son tratadas en este trabajo, de las cuales dos pertenecen al infraorden Nepomorpha: Corixidae: Heterocorixa y Notonectidae: Martarega; 16 pertenecen a Gerromorpha: de las cuales 12 pertenecen a la familia Gerridae: Metrobates, Tachygerris, Limnogonus (dos especies), Brachymetra, Neogerris, Potamobates, Trepobates (tres especies), Telmatometra, Rheumatobates; tres pertenecen a la familia Veliidae: Rhagovelia, Microvelia (dos especies), y una a Mesoveliidae: Mesovelia. Palabras clave: Insectos acuáticos. Corixidae. Notonectidae. Gerridae. Veliidae. Introduction this region is considered of the utmost importance for conservation strategies, potential management options, and During the last years, the knowledge about aquatic increase environmental sustainability (Porro et al. -
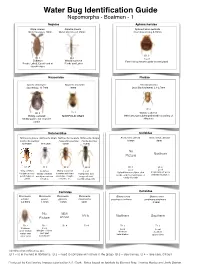
Water Bug ID Guide
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants.