Australian Statistics on Medicines 2003
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Deliverable 5.A Interim Report on the Study Results APPENDIX 2
Deliverable 5.a Interim report on the study results APPENDIX 2: Algorithms used to identify study variables for service contract EMA/2011/38/CN ‐ PIOGLITAZONE November 28th 2012 D5.a Interim report on the study results (Appendix 2) for Service Contract EMA/2011/38/CN PIOGLITAZONE Author(s): Vera Ehrenstein (AUH‐AS) APPENDIX 2. ALGORITHMS USED TO IDENTIFY STUDY VARIABLES Algorithms for AU Database DISEASE/CONDITION ICD-8 CODE (1977-1993) ICD-10 CODE (1994-) Diabetes type 2 250.00; 250.06; 250.07; 250.09 E11.0; E11.1; E11.9 Cancer of bladder 188 C67 Haematuria N/A R31 Haematuria, unspecified B18, K70.0–K70.3, K70.9, K71, K73, Mild hepatic impairment 571, 573.01, 573.04 K74, K76.0 Moderate to severe hepatic 070.00, 070.02, 070.04, 070.06, B15.0, B16.0, B16.2, B19.0, K70.4, impairment 070.08, 573.00, 456.00–456.09 K72, K76.6, I85 Acute myocardial infarction 410 I21-I23 Acute coronary syndrome 410, 413 I20-I24 Ischemic heart disease 410-414 I20-I25 427.09, 427.10, 427.11, 427.19, Congestive heart failure I50, I11.0, I13.0,I13.2 428.99, 782.49; Acute renal failure N/A N17 Diabetic coma N/A E10.0, E11.0, E12.0,E13.0, E14.0 Diabetic acidosis N/A E10.1, E11.1, E12.1,E13.1, E14.1 F10.1-F10.9, G31.2, G62.1, G72.1, Alcoholism 291, 303, 577.10, 571.09, 571.10 I42.6, K29.2, K86.0, Z72.1 Obesity 277.99 E65-E66 D5.a Interim report on the study results (Appendix 2) for Service Contract EMA/2011/38/CN PIOGLITAZONE Author(s): Vera Ehrenstein (AUH‐AS) Algorithms for defining acute events in Denmark, ICD-10 code Event ICD-10 code I21.x, I23.x http://apps.who.int/classifications/icd10/browse/2010/en#/I21 -

Combination of Pretreatments with Acetic Acid and Sodium Methoxide for Efficient Digoxin Preparation from Digitalis Glycosides in Digitalis Lanata Leaves
Pharmacology & Pharmacy, 2016, 7, 200-207 Published Online May 2016 in SciRes. http://www.scirp.org/journal/pp http://dx.doi.org/10.4236/pp.2016.75026 Combination of Pretreatments with Acetic Acid and Sodium Methoxide for Efficient Digoxin Preparation from Digitalis Glycosides in Digitalis lanata Leaves Yasuhiko Higashi*, Yukari Ikeda, Youichi Fujii Department of Analytical Chemistry, Faculty of Pharmaceutical Sciences, Hokuriku University, Kanazawa, Japan Received 21 April 2016; accepted 28 May 2016; published 31 May 2016 Copyright © 2016 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract We previously developed an HPLC method for determination of lanatoside C, digoxin and α-acetyl- digoxin in digitalis glycosides isolated from Digitalis lanata leaves. Here, we present an improved HPLC-UV method to determine those compounds and deslanoside. We used the improved method to examine the effects of various pretreatments on the amounts of the four compounds isolated from the leaves, with the aim of maximizing the yield of digoxin. Leaves were extracted with 50% methanol, followed by clean-up on a Sep-Pak C18 cartridge prior to HPLC analysis. The amounts of lanatoside C, digoxin and α-acetyldigoxin per 100 mg of the leaves without pretreatment were 115.6, 7.45 and 23.8 μg, respectively (deslanoside was not detected). Pretreatment with acetic ac- id, which activated deglucosylation mediated by digilanidase present in the leaves, increased the amounts of digoxin and α-acetyldigoxin, while lanatoside C and deslanoside were not detected. Pretreatment with sodium methoxide, which hydrolyzed lanatoside C to deslanoside, increased the yields of deslanoside and digoxin, while lanatoside C and α-acetyldigoxin were not detected. -

Marie Louise De Bruin Drug Induced Arrhythmias
Marie Louise De Bruin drug induced arrhythmias Quantifying the problem Cover design: Tom Frantzen CIP-gegevens Koninklijke Bibliotheek, Den Haag De Bruin, Marie Louise Drug-induced arrhythmias, quantifying the problem / Marie Louise De Bruin Thesis Utrecht - with ref.- with summary in Dutch ISBN: 90-808203-3-4 © Marie Louise De Bruin drug-induced arrhythmias, quantifying the problem Geneesmiddel-geïnduceerde hartritmestoornissen, kwantificering van het probleem (met een samenvatting in het Nederlands) Proefschrift ter verkrijging van de graad van doctor aan de Universiteit van Utrecht op gezag van de Rector Magnificus Prof. dr W.H. Gispen, ingevolge het besluit van het College voor Promoties in het openbaar te verdedigen op woensdag 1 december 2004 des namiddags om 14.30 uur door Marie Louise De Bruin Geboren op 1 april 1974 te Haarlem promotores Prof. dr H.G.M. Leufkens Utrecht Institute for Pharmaceutical Sciences (UIPS), Department of Pharmaco- epidemiology and Pharmacotherapy, Utrecht University, Utrecht, the Netherlands Prof. dr A.W. Hoes Julius Center for Health Sciences and Primary Care, University Medical Center, Utrecht, the Netherlands The work in this thesis was performed at the Department of Pharmacoepidemiology and Pharmacotherapy of the Utrecht Institute for Pharmaceutical Sciences (Utrecht) and the Julius Center for Health Sciences and Primary Care (Utrecht), in collabo- ration with the PHARMO Institute (Utrecht), the Netherlands Pharmacovigilance Centre Lareb (‘s-Hertogenbosch), the WHO-Uppsala Monitoring Centre (Uppsala, Sweden) and the Academic Medical Center (Amsterdam). The research presented in this thesis was funded by the Utrecht Institute for Pharmaceutical Sciences, and an unrestricted grant from the Dutch Medicines Evaluation Board. The study presented in chapter 3.1 was funded by an unrestricted grant from Janssen Pharmaceutica NV, Beerse, Belgium. -
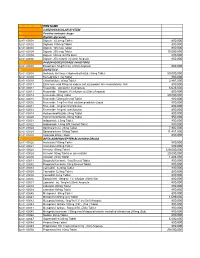
National Code Item Name 1
NATIONAL CODE ITEM NAME 1 CARDIOVASCULAR SYSTEM 1A Positive inotropic drugs 1AA Digtalis glycoside 02-01-00001 Digoxin 62.5mcg Tablet 800,000 02-01-00002 Digitoxin 100mcg Tablet 800,000 02-01-00003 Digoxin 125 mcg Tablet 800,000 02-01-00004 Digoxin 250 mcg Tablet 15,000,000 02-01-00005 Digoxin 50mcg /ml PG Elixir 800,000 02-01-00006 Digoxin 250 mcg/ml inj (2ml) Ampoule 800,000 1AB PHOSPHODIESTERASE INHIBITORS 02-01-00007 Enoximone 5mg/1ml inj (20ml) Ampoule 800,000 1B DIURETICS 02-01-00008 Amiloride Hcl 5mg + Hydrochlorthiazide 50mg Tablet 50,000,000 02-01-00009 Bumetanide 1 mg Tablet 800,000 02-01-00010 Chlorthalidone 50mg Tablet 2,867,000 02-01-00011 Ethacrynic acid 50mg as sodium salt inj (powder for reconstitution) Vial 800,000 02-01-00012 Frusemide 20mg/2ml inj Ampoule 6,625,000 02-01-00013 Frusemide 10mg/ml,I.V.infusion inj (25ml) Ampoule 800,000 02-01-00014 Frusemide 40mg Tablet 20,000,000 02-01-00015 Frusemide 500mg Scored Tablet 800,000 02-01-00016 Frusemide 1mg/1ml Oral solution peadiatric Liquid 800,000 02-01-00017 Frusemide 4mg/ml Oral Solution 800,000 02-01-00018 Frusemide 8mg/ml oral Solution 800,000 02-01-00019 Hydrochlorothiazide 25mg Tablet 800,000 02-01-00020 Hydrochlorothiazide 50mg Tablet 950,000 02-01-00021 Indapamide 2.5mg Tablet 800,000 02-01-00022 Indapamide 1.5mg S/R Coated Tablet 800,000 02-01-00023 Spironolactone 25mg Tablet 7,902,000 02-01-00024 Spironolactone 100mg Tablet 11,451,000 02-01-00025 Xipamide 20mg Tablet 800,000 1C BETA-ADRENOCEPTER BLOCKING DRUGS 02-01-00026 Acebutolol 100mg Tablet 800,000 02-01-00027 Acebutolol 200mg Tablet 800,000 02-01-00028 Atenolol 100mg Tablet 120,000,000 02-01-00029 Atenolol 50mg Tablet or (scored tab) 20,000,000 02-01-00030 Atenolol 25mg Tablet 1,483,000 02-01-00031 Bisoprolol fumarate 5mg Scored Tablet 800,000 02-01-00032 Bisoprolol fumarate 10mg Scored Tablet 800,000 02-01-00033 Carvedilol 6.25mg Tablet 800,000 02-01-00034 Carvedilol 12.5mg Tablet 800,000 02-01-00035 Carvedilol 25mg Tablet 800,000 02-01-00036 Esmolol Hcl 10mg/ml I.V. -

WO 2012/148799 Al 1 November 2012 (01.11.2012) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2012/148799 Al 1 November 2012 (01.11.2012) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 9/107 (2006.01) A61K 9/00 (2006.01) kind of national protection available): AE, AG, AL, AM, A 61 47/10 (2006.0V) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, PCT/US2012/034361 HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, (22) International Filing Date: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 20 April 2012 (20.04.2012) MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (26) Publication Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/480,259 28 April 201 1 (28.04.201 1) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, (71) Applicant (for all designated States except US): BOARD UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, OF REGENTS, THE UNIVERSITY OF TEXAS SYS¬ TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, TEM [US/US]; 201 West 7th St., Austin, TX 78701 (US). -

Time in Therapeutic Range and Outcomes After Warfarin Initiation in Newly Diagnosed Atrial Fibrillation Patients with Renal Dysfunction
http://www.diva-portal.org This is the published version of a paper published in Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease. Citation for the original published paper (version of record): Szummer, K., Gasparini, A., Eliasson, S., Ärnlöv, J., Qureshi, A R. et al. (2017) time in therapeutic range and outcomes after warfarin initiation in newly diagnosed atrial fibrillation patients with renal dysfunction. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease, 6(3): e004925 https://doi.org/10.1161/JAHA.116.004925 Access to the published version may require subscription. N.B. When citing this work, cite the original published paper. Permanent link to this version: http://urn.kb.se/resolve?urn=urn:nbn:se:du-24516 ORIGINAL RESEARCH Time in Therapeutic Range and Outcomes After Warfarin Initiation in Newly Diagnosed Atrial Fibrillation Patients With Renal Dysfunction Karolina Szummer, MD, PhD; Alessandro Gasparini, MSc; Staffan Eliasson, MD; Johan Arnl€ ov,€ MD, PhD; Abdul Rashid Qureshi, MD, PhD; Peter Barany, MD, PhD; Marie Evans, MD, PhD; Leif Friberg, MD, PhD; Juan Jesus Carrero, PharmMed, PhD Background-—It is unknown whether renal dysfunction conveys poor anticoagulation control in warfarin-treated patients with atrial fibrillation and whether poor anticoagulation control associates with the risk of adverse outcomes in these patients. Methods and Results-—This was an observational study from the Stockholm CREatinine Measurements (SCREAM) cohort including all newly diagnosed atrial fibrillation patients initiating treatment with warfarin (n=7738) in Stockholm, Sweden, between 2006 and 2011. Estimated glomerular filtration rate (eGFR; mL/min per 1.73 m2) was calculated from serum creatinine. -

Evaluating Onco-Geriatric Scores and Medication Risks to Improve Cancer Care for Older Patients
Evaluating onco-geriatric scores and medication risks to improve cancer care for older patients Dissertation zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn vorgelegt von IMKE ORTLAND aus Quakenbrück Bonn 2019 Angefertigt mit Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn. Diese Dissertation ist auf dem Hochschulschriftenserver der ULB Bonn elektronisch publiziert. https://nbn-resolving.org/urn:nbn:de:hbz:5-58042 Erstgutachter: Prof. Dr. Ulrich Jaehde Zweitgutachter: Prof. Dr. Andreas Jacobs Tag der Promotion: 28. Februar 2020 Erscheinungsjahr: 2020 Danksagung Auf dem Weg zur Promotion haben mich viele Menschen begleitet und in ganz unterschiedlicher Weise unterstützt. All diesen Menschen möchte ich an dieser Stelle ganz herzlich danken. Mein aufrichtiger Dank gilt meinem Doktorvater Prof. Dr. Ulrich Jaehde für das in mich gesetzte Vertrauen, sowie für die Überlassung dieses spannenden Dissertationsthemas. Die uneingeschränkte Unterstützung, wertvollen Diskussionen und die mitreißende Begeisterung für die Wissenschaft haben mich während aller Phasen der Dissertation stets motiviert, unterstützt und sehr viel Wertvolles gelehrt. Prof. Dr. Andreas Jacobs danke ich herzlich für die Initiierung dieses interessanten Projekts, für die stetige Begeisterung und Unterstützung, sowie für das mir entgegengebrachte Vertrauen. Die ausgezeichnete Zusammenarbeit mit dem Johanniter Krankenhaus Bonn hat ganz maßgeblich zum Gelingen dieser Arbeit beigetragen. Auch danke ich Prof. Dr. Andreas Jacobs herzlich für die Bereitschaft, das Koreferat dieser Arbeit zu übernehmen. Ebenfalls danke ich herzlich Prof. Dr. Yon-Dschun Ko für seine fortwährende Motivation und seinen Einsatz, sowie für das mir geschenkte Vertrauen, dieses Projekt am Johanniter Krankenhaus zu realisieren. Ebenfalls danke ich Prof. -

Quality Issues in Caring for Older People
Doctoral Thesis - Tesis Doctoral Quality issues in caring for older people: • Appropriateness of transition from long-term care facilities to acute hospital care • Potentially inappropriate medication: development of a European list Anna Renom Guiteras Prof. Gabriele Meyer Prof. Ramón Miralles Basseda Martin Luther University Halle-Wittenberg Universitat Autònoma de Barcelona Halle (Saale) & Barcelona, Catalonia University of Witten/Herdecke Spain Witten Germany Programa de doctorat en Medicina Departament de Medicina, Facultat de Medicina Universitat Autònoma de Barcelona Barcelona, 2015 13 Contents 15 1. Introduction • Research context • Background of the research topics • Pesetaio of the ailes 23 2. Summary and discussion of the results 31 3. Conclusions 37 4. References 47 5. Articles • Article 1: Renom-Guiteras A, Uhrenfeldt L, Meyer G, Mann E. Assessment tools for determining appropriateness of admission to acute care of persons transferred from long-term care facilities: a systematic review. BMC Geriatr. 2014;14:80 • Article 2: Renom-Guiteras A, Meyer G, Thürmann PA. The EU(7)-PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol. 2015;71(7):861-75 77 6. Annexes • Annex 1.1 (article 1) - Additional file 1: Studies dealing with assessment tools for determining appropriateness of hospital admissions among residents of LTC facilities. • Annex 1.2 (article 1) - Additional file 2: Characteristics of the assessment tools for determining appropriateness of hospital admissions among residents of LTC facilities. • Annex 2.1 (article 2) - Appendix 1: Complete EU(7)-PIM list • Annex 2.2 (article 2) - Appendix 2: Questionable Potentially Inappropriate Medications (Questionable PIM): results of the Delphi survey. -

Silibinin Component for the Treatment of Hepatitis Silbininkomponente Zur Behandlung Von Hepatitis Composant De Silibinine Pour Le Traitement De L’Hépatite
(19) & (11) EP 2 219 642 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 31/357 (2006.01) A61P 1/16 (2006.01) 21.09.2011 Bulletin 2011/38 A61P 31/12 (2006.01) (21) Application number: 08849759.9 (86) International application number: PCT/EP2008/009659 (22) Date of filing: 14.11.2008 (87) International publication number: WO 2009/062737 (22.05.2009 Gazette 2009/21) (54) SILIBININ COMPONENT FOR THE TREATMENT OF HEPATITIS SILBININKOMPONENTE ZUR BEHANDLUNG VON HEPATITIS COMPOSANT DE SILIBININE POUR LE TRAITEMENT DE L’HÉPATITE (84) Designated Contracting States: (56) References cited: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR WO-A-02/067853 GB-A- 2 167 414 HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT RO SE SI SK TR • POLYAK STEPHEN J ET AL: "Inhibition of T- cell inflammatory cytokines, hepatocyte NF-kappa B (30) Priority: 15.11.2007 EP 07022187 signaling, and HCV infection by standardized 15.11.2007 US 988168 P silymarin" GASTROENTEROLOGY, vol. 132, no. 25.03.2008 EP 08005459 5, May 2007 (2007-05), pages 1925-1936, XP002477920 ISSN: 0016-5085 (43) Date of publication of application: • MAYER K E ET AL: "Silymarin treatment of viral 25.08.2010 Bulletin 2010/34 hepatitis: A systematic review" JOURNAL OF VIRAL HEPATITIS 200511 GB, vol. 12, no. 6, (60) Divisional application: November 2005 (2005-11), pages 559-567, 11005445.9 XP002477921 ISSN: 1352-0504 1365-2893 • CHAVEZ M L: "TREATMENT OF HEPATITIS C (73) Proprietor: Madaus GmbH WITH MILK THISTLE?" JOURNAL OF HERBAL 51067 Köln (DE) PHARMACOTHERAPY, HAWORTH HERBAL PRESS, BINGHAMTON, US, vol. -

Management of Graves Disease:€€A Review
Clinical Review & Education Review Management of Graves Disease A Review Henry B. Burch, MD; David S. Cooper, MD Author Audio Interview at IMPORTANCE Graves disease is the most common cause of persistent hyperthyroidism in adults. jama.com Approximately 3% of women and 0.5% of men will develop Graves disease during their lifetime. Supplemental content at jama.com OBSERVATIONS We searched PubMed and the Cochrane database for English-language studies CME Quiz at published from June 2000 through October 5, 2015. Thirteen randomized clinical trials, 5 sys- jamanetworkcme.com and tematic reviews and meta-analyses, and 52 observational studies were included in this review. CME Questions page 2559 Patients with Graves disease may be treated with antithyroid drugs, radioactive iodine (RAI), or surgery (near-total thyroidectomy). The optimal approach depends on patient preference, geog- raphy, and clinical factors. A 12- to 18-month course of antithyroid drugs may lead to a remission in approximately 50% of patients but can cause potentially significant (albeit rare) adverse reac- tions, including agranulocytosis and hepatotoxicity. Adverse reactions typically occur within the first 90 days of therapy. Treating Graves disease with RAI and surgery result in gland destruction or removal, necessitating life-long levothyroxine replacement. Use of RAI has also been associ- ated with the development or worsening of thyroid eye disease in approximately 15% to 20% of patients. Surgery is favored in patients with concomitant suspicious or malignant thyroid nodules, coexisting hyperparathyroidism, and in patients with large goiters or moderate to severe thyroid Author Affiliations: Endocrinology eye disease who cannot be treated using antithyroid drugs. -

Comparative Genomics of the Major Parasitic Worms
Comparative genomics of the major parasitic worms International Helminth Genomes Consortium Supplementary Information Introduction ............................................................................................................................... 4 Contributions from Consortium members ..................................................................................... 5 Methods .................................................................................................................................... 6 1 Sample collection and preparation ................................................................................................................. 6 2.1 Data production, Wellcome Trust Sanger Institute (WTSI) ........................................................................ 12 DNA template preparation and sequencing................................................................................................. 12 Genome assembly ........................................................................................................................................ 13 Assembly QC ................................................................................................................................................. 14 Gene prediction ............................................................................................................................................ 15 Contamination screening ............................................................................................................................ -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01