USMPAKL00310919(1) AKLIEF Patient Brochure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

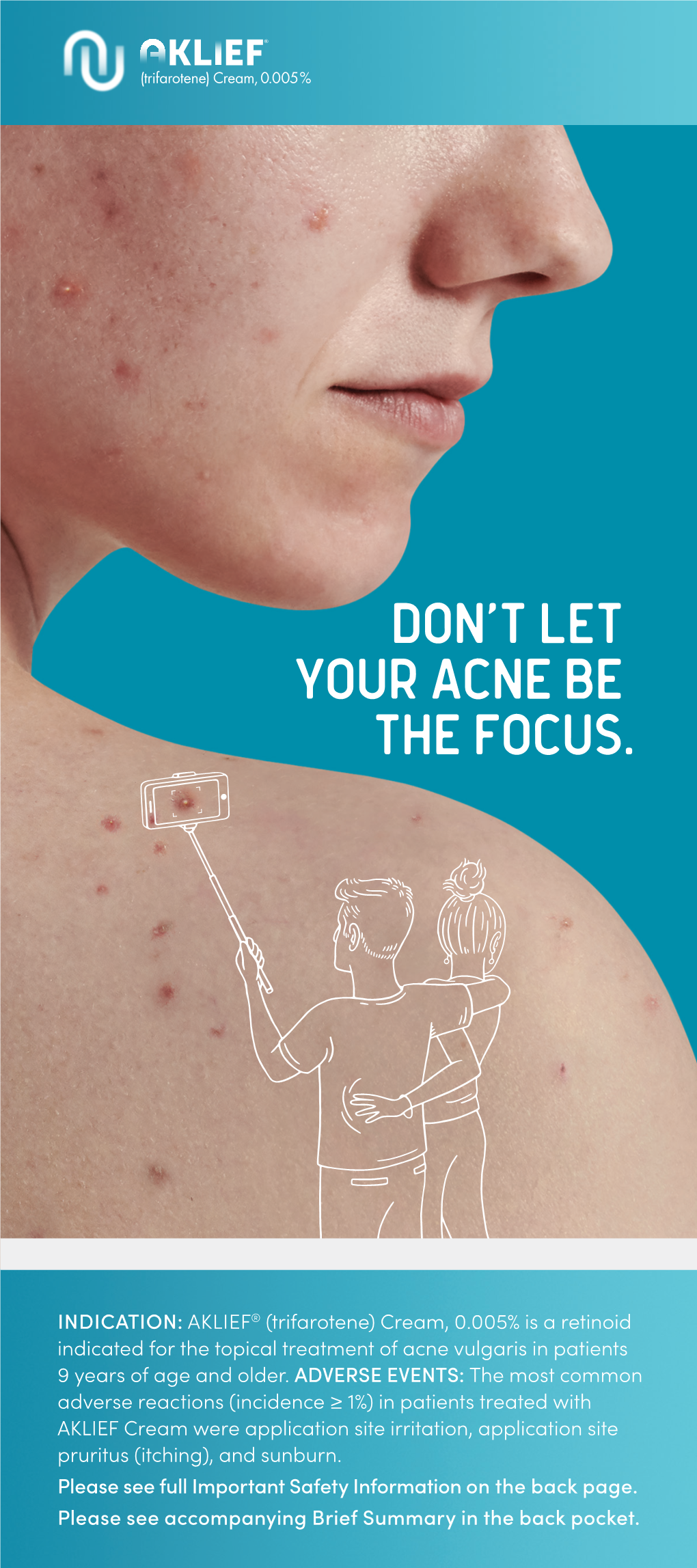
What Is Acne? Acne Is a Disease of the Skin's Sebaceous Glands
What is Acne? Acne is a disease of the skin’s sebaceous glands. Sebaceous glands produce oils that carry dead skin cells to the surface of the skin through follicles. When a follicle becomes clogged, the gland becomes inflamed and infected, producing a pimple. Who Gets Acne? Acne is the most common skin disease. It is most prevalent in teenagers and young adults. However, some people in their forties and fifties still get acne. What Causes Acne? There are many factors that play a role in the development of acne. Some of these include hormones, heredity, oil based cosmetics, topical steroids, and oral medications (corticosteroids, lithium, iodides, some antiepileptics). Some endocrine disorders may also predispose patients to developing acne. Skin Care Tips: Clean skin gently using a mild cleanser at least twice a day and after exercising. Scrubbing the skin can aggravate acne, making it worse. Try not to touch your skin. Squeezing or picking pimples can cause scars. Males should shave gently and infrequently if possible. Soften your beard with soap and water before putting on shaving cream. Avoid the sun. Some acne treatments will cause skin to sunburn more easily. Choose oil free makeup that is “noncomedogenic” which means that it will not clog pores. Shampoo your hair daily especially if oily. Keep hair off your face. What Makes Acne Worse? The hormone changes in females that occur 2 to 7 days prior to period starting each month. Bike helmets, backpacks, or tight collars putting pressure on acne prone skin Pollution and high humidity Squeezing or picking at pimples Scrubs containing apricot seeds. -

What Is Acne and Why Do I Have Pimples?
What is acne and why do I have pimples? The medical term for “pimples” is acne. Most people develop at least some acne, especially during their teenage years. Although many believe that acne comes from being dirty, this is not true; rather, acne is the result of changes that occur during puberty. Your skin is made of layers. To keep the skin from getting dry, the skin makes oil in little wells called “sebaceous glands” that are found in the deeper layers of the skin. “Whiteheads” or “blackheads” are clogged sebaceous glands. “Blackheads” are not caused by dirt blocking the pores, but rather by oxidation (a chemical reaction that occurs when the oil reacts with oxygen in the air). People with acne have glands that make more oil and are more easily plugged, causing the glands to swell. Hormones, bacteria (called P. acnes) and your family’s likelihood to have acne (genetic susceptibility) also play a role. SKIN HYGIENE Good skin care habits are important and support the medications your doctor prescribes for your acne. » Wash your face twice a day, once in the morning and once in the evening (which includes any showers you take). » Avoid over-washing/over-scrubbing your face as this will not improve the acne and may lead to dryness and irritation, which can interfere with your medications. » In general, milder soaps and cleansers are better for acne-prone skin. The soaps labeled “for sensitive skin” are milder than those labeled “deodorant soap” or “antibacterial soap.” » Many “acne washes” may contain salicylic acid. Salicylic acid (SA) fights oil and bacteria mildly but can be drying and can add to irritation. -

Sunburn, Suntan and the Risk of Cutaneous Malignant Melanoma the Western Canada Melanoma Study J.M
Br. J. Cancer (1985), 51, 543-549 Sunburn, suntan and the risk of cutaneous malignant melanoma The Western Canada Melanoma Study J.M. Elwood1, R.P. Gallagher2, J. Davison' & G.B. Hill3 1Department of Community Health, University of Nottingham, Queen's Medical Centre, Nottingham NG7 2UH, UK; 2Division of Epidemiology and Biometry, Cancer Control Agency of British Columbia, 2656 Heather Street, Vancouver BC, Canada V5Z 3J3; and 3Department of Epidemiology, Alberta Cancer Board, Edmonton, Alberta, Canada T6G OTT2. Summary A comparison of interview data on 595 patients with newly incident cutaneous melanoma, excluding lentigo maligna melanoma and acral lentiginous melanoma, with data from comparison subjects drawn from the general population, showed that melanoma risk increased in association with the frequency and severity of past episodes of sunburn, and also that melanoma risk was higher in subjects who usually had a relatively mild degree of suntan compared to those with moderate or deep suntan in both winter and summer. The associations with sunburn and with suntan were independent. Melanoma risk is also increased in association with a tendency to burn easily and tan poorly and with pigmentation characteristics of light hair and skin colour, and history freckles; the associations with sunburn and suntan are no longer significant when these other factors are taken into account. This shows that pigmentation characteristics, and the usual skin reaction to sun, are more closely associated with melanoma risk than are sunburn and suntan histories. -

DRUG-INDUCED PHOTOSENSITIVITY (Part 1 of 4)
DRUG-INDUCED PHOTOSENSITIVITY (Part 1 of 4) DEFINITION AND CLASSIFICATION Drug-induced photosensitivity: cutaneous adverse events due to exposure to a drug and either ultraviolet (UV) or visible radiation. Reactions can be classified as either photoallergic or phototoxic drug eruptions, though distinguishing between the two reactions can be difficult and usually does not affect management. The following criteria must be met to be considered as a photosensitive drug eruption: • Occurs only in the context of radiation • Drug or one of its metabolites must be present in the skin at the time of exposure to radiation • Drug and/or its metabolites must be able to absorb either visible or UV radiation Photoallergic drug eruption Phototoxic drug eruption Description Immune-mediated mechanism of action. Response is not dose-related. More frequent and result from direct cellular damage. May be dose- Occurs after repeated exposure to the drug dependent. Reaction can be seen with initial exposure to the drug Incidence Low High Pathophysiology Type IV hypersensitivity reaction Direct tissue injury Onset >24hrs <24hrs Clinical appearance Eczematous Exaggerated sunburn reaction with erythema, itching, and burning Localization May spread outside exposed areas Only exposed areas Pigmentary changes Unusual Frequent Histology Epidermal spongiosis, exocytosis of lymphocytes and a perivascular Necrotic keratinocytes, predominantly lymphocytic and neutrophilic inflammatory infiltrate dermal infiltrate DIAGNOSIS Most cases of drug-induced photosensitivity can be diagnosed based on physical examination, detailed clinical history, and knowledge of drug classes typically implicated in photosensitive reactions. Specialized testing is not necessary to make the diagnosis for most patients. However, in cases where there is no prior literature to support a photosensitive reaction to a given drug, or where the diagnosis itself is in question, implementing phototesting, photopatch testing, or rechallenge testing can be useful. -

SUNBURN QUESTIONS and ANSWERS Don’T Fry Day
www.skincancerprevention.org SUNBURN QUESTIONS AND ANSWERS Don’t Fry Day Don’t Fry Day Sunburn Questions and Answers Why is it important to avoid sunburn on Don’t Fry Day - and every day? Sunburns are painful, and should be avoided for that reason. But sunburns represent skin damage. Your skin can repair some damage to DNA that results from too much sun, but this safety mechanism can be overwhelmed by the massive DNA damage caused by a sunburn. Mutations that are not repaired can lead to the development of skin cancer. This is why we should all avoid sunburn, especially for children. But wait, there’s more: sun’s skin-damaging ultraviolet (UV) rays also produce free radicals in skin cells, which can lead not only to cancer but also to wrinkles, blotches, and premature aging. If Don’t Fry Day is cloudy, I don’t need to take any preCautions, right? In fact, some of the worst sunburns happen on hazy days. Many people mistakenly assume that if it’s cool or cloudy outdoors they won’t get burned. They don’t realize that while clouds block the heat (infrared) energy, UV rays can still penetrate through quite strongly. What are other Causes of sunburn? Probably the most common cause of sunburn is accidental overexposure. Falling asleep while in the sun, forgetting to apply or re-apply sunscreen, or underestimating how quickly your skin will burn are typical mistakes. There may be no signs or symptoms while the overexposure is occurring because it can take hours following the exposure before the skin becomes red or tender. -

WHAT YOU NEED to KNOW ABOUT SEYSARA® a Novel Treatment Developed Specifi Cally for Acne
Not an actual patient, results may vary. WHAT YOU NEED TO KNOW ABOUT SEYSARA® A novel treatment developed specifi cally for acne. PLEASE SEE THE ACCOMPANYING PATIENT INFORMATION AND FULL PRESCRIBING INFORMATION. almirall.us INTRODUCING SEYSARA: A NOVEL ORAL ANTIBIOTIC TREATMENT DESIGNED SPECIFICALLY FOR ACNE WHAT IS SEYSARA? WHAT CAUSES ACNE? SEYSARA is a prescription medicine used to treat moderate to Acne appears when a small hole in our skin (pore) clogs with dead severe acne vulgaris in people 9 years and older. SEYSARA should not skin cells. Normally, dead skin cells rise to the surface of the pore, be used for the treatment or prevention of infections. It is not known where they are shed. Excess production of sebum—the oil that keeps if SEYSARA is safe and effective for use for longer than 12 weeks. our skin from drying out—can cause the dead skin cells to stick SEYSARA should not be used in children under 9 years of age, or if you together and get trapped inside the pore. 1 are pregnant or breastfeeding. Sometimes the bacteria that live naturally on our skin, C. acnes, also get inside the pore, where they can multiply quickly. With WHAT IS MODERATE TO SEVERE ACNE? bacteria inside, the pore becomes infl amed (red and swollen). If the acne goes deep into the skin, an acne cyst or nodule appears.4 Acne is a common skin condition involving blockage and/or infl ammation of hair follicles and their associated gland. Depending on the severity, acne is generally categorized as mild, moderate, or severe. -

Tanning? How Last Millennium Elizabeth Michaelson to Learn Whether Tanning’S Popu- Have to Clear Up
BEAUTY & ANTI-AGING Tanning? How Last Millennium ELIZABETH MICHAELSON To learn whether tanning’s popu- have to clear up. A healthy glow means larity has faded, we went right to the your [natural] skin tone, glowing. You, source: fashion and beauty magazines. looking luminous.” After an informal focus group of eight Larkworthy agrees. “Skin that’s not prominent beauty editors confirmed that tan is gorgeous… The more you take suntans are at last going out of style, we care of it and don’t subject it to the sun, conducted several in-depth follow-up the less makeup you need. Taking care Lois Johnson Valerie Monroe interviews. As Jane Larkworthy, Beauty of your skin is almost, in and of itself, a More Magazine O, The Oprah Magazine Director of W, notes, “I can’t remember type of foundation. It’s one step less in the last time I saw a tanned model in my your makeup routine — you don’t have magazine; I can’t remember the last time to cover up the wrinkles as much as you I saw a tanned model on the runway.” would if you’d spent 20 or 30 years in Tanning’s TIRED TYRANNY Val Monroe, O, The Oprah Magazine’s the sun.” Suntans promote a particularly Beauty Director, has likewise observed limited vision of health and beauty, consumers’ shifting attitudes: “I do no- YOU GLOW, GIRL since the popular image of the tanned tice more people walking around pale in But while UV tan-seeking is a no-no, all-American beauty was tradition- the summertime than there used to be.” a little glow, the kind that comes ally a light-skinned Caucasian whose Lois Johnson, Beauty Editor at More, from non-UV tanning products and golden glow was the result of UV puts it more flatly: “Tanning as a life bronzers, while still using sunscreen, exposure rather than genetic inheri- priority is over.” is still permissible and won’t sacrifice tance. -

Sun Protection After a Burn Injury
Sun Protection After a Burn Injury September 2018 www.msktc.org/burn/factsheets BURN Fact Sheet This fact sheet explains the The sun has many beneficial properties, but the sun emits three types of ultraviolet (UV) light that can importance of sun harm skin and has been linked to skin cancers (basal and squamous carcinoma, melanoma) and vision protection as you recover problems (cataracts and macular degeneration). Healed burns, donor sites and skin grafts are more and heal from a burn injury. sensitive to sunlight. It describes how sun exposure affects your skin, Your Burn Injury and Sun Exposure ways to limit sun exposure, and offers resources to Burn skin sensitivity. Healed burns or skin grafts may be extremely sensitive to sunlight and may sunburn learn more about protecting more severely even after short periods of time in the sun compared to before your injury. Sun sensitivity yourself after a burn injury may last for a year or more. In addition, some medications can cause you to be more sensitive to the sun. Pigmentation. The color of our skin is related to the amount of melanin that each of us has in our skin. When someone has a second degree or deeper burn injury, the pigment of the skin is affected because melanin is located in the epidermis (the outer most layer of skin). With a burn injury, that pigment is lost. With healing, the pigment may return, but this process is unpredictable. Often, newly healed skin appears pink and unpigmented. As the scar matures, the skin may regain pigment. -

Skin Cancer Attacks More Than One Million Americans Every Year, And
Skin Preventing Sun Damage SKIN CANCER: ARE The easiest way to prevent skin damage and lessen your cancer chances of getting skin cancer is to avoid getting sunburn. attacks YOU SAFE IN THE Here are a few tips to help keep you safe in the sun: more than • Stay out of the sun between 10 a.m. and 4 p.m. when it one million SUN? is strongest. • Wear clothes with tightly woven fabric and a hat that Americans Many people describe sun-tanned skin as a “healthy glow,” shades your face, neck and ears. but it’s not exactly the truth. There is no such thing as a every healthy tan. • Wear sunglasses whenever you are outside to avoid year, and developing cataracts and damaging your retinas. Sun Damage • Use sunscreen that has at least 15 SPF every day, exposure Any type of suntan is the result of sun damage caused by especially on your lips and the tips of your ears and nose. to the sun exposure to ultraviolet (UV) radiation from the sun. Other types of sun damage include wrinkles, age spots, freckles, • Avoid using tanning beds. Tanning beds give off causes tough or leathery skin, dilated blood vessels, sunburn and radiation that is 10 to 15 times stronger than the sun. skin cancer. over 90 • Protect children from sun damage. Most sun exposure The sun emits two types of UV radiation: UVA (which occurs before age 18. percent of causes aging) and UVB (which causes burning). Both UVA and UVB rays are undetectable to a person sitting in the Avoiding excessive sun exposure and sunburn is the best skin sun—you cannot feel them on your skin. -

Sun Safety and Protection Tips American Academy of Pediatrics
Sun Safety and Protection Tips American Academy of Pediatrics Spending time outdoors is a common activity on spring breaks or summer vacations, but remember to protect against the sun’s rays. Everyone is at risk for sunburn. Children especially need to be protected from the sun’s burning rays, since most sun damage occurs in childhood. Like other burns, sunburn will leave the skin red, warm, and painful. In severe cases, it may cause blistering, fever, chills, headache, and a general feeling of illness. The American Academy of Pediatrics offers tips to keep children safe in the sun. Sun Safety for Babies Under 6 Months Babies under 6 months of age should be kept out of direct sunlight. Move your baby to the shade under a tree, umbrella or stroller canopy. Dress babies in lightweight clothing that covers the arms and legs and use brimmed hats that shade the neck to prevent sunburn. When adequate clothing and shade are not available, parents can apply a minimal amount of sunscreen with at least 15 SPF (sun protection factor) on infants under 6 months to small areas, such as the infant's face and the back of the hands. Remember it takes 30 minutes to be effective. If an infant gets sunburn, apply cool compresses to the affected area. Sun Safety for Kids The first, and best, line of defense against harmful ultraviolet radiation (UVR) exposure is covering up. Stay in the shade whenever possible, and limit sun exposure during the peak intensity hours - between 10 a.m. and 4 p.m. -

Facts and Myths: Ultraviolet Light and Sun Protection
Facts and Myths: Ultraviolet Light and Sun Protection Katherine T. Steele, MD Assistant Professor of Clinical Dermatology University of Pennsylvania Fundamentals: Ultraviolet light Sunlight consists of 2 types of ultraviolet (UV) light • UVA • UVB • UVC (absorbed by the ozone in the earth’s atmosphere) Fundamentals: Ultraviolet light UVA rays • Premature aging – Dark spots – Wrinkles – Loose skin UVB rays • Sunburn Both UVA + UVB • Skin cancer Sun Protection: Facts What is the most effective way to protect your skin from the sun? 1) Avoiding sun exposure 2) Sun-protective clothing 3) Sunscreen Sun Avoidance UVB intensity peaks from 10 AM to 2 PM Note: UVA intensity is relatively constant throughout the day Avoid Peak Hours 6 AM 10 AM 2 PM 8 PM Which is the most effective way to protect your skin from the sun? 1) Avoiding sun exposure 2) Sun-protective clothing 3) Sunscreen Sun-protective clothing with UPF (ultraviolet protection factor) Benefits of sun-protective clothing • Once garment is on, it starts working immediately • Won’t wear off throughout the day • Not messy, oily or greasy • Non-allergenic • Potential cost savings: garments last many seasons • Created for Leisure and Sporting activities • Fashionable and trendy https://yoursummerskin.com/pages/about-upf-clothing Sun-Protective Clothing Sun Threadz Colleen Dougherty Bronstein Supporter of Melanoma Program at the Abramson Cancer Center of the University of Pennsylvania Which is the most effective way to protect your skin from the sun? 1) Avoiding sun exposure 2) Sun-protective clothing 3) Sunscreen Sunscreen: Facts Sunscreen labeling Broad-spectrum = Provides protection against both UVA + UVB Only broad-spectrum sunscreens with SPF 15+ can claim to reduce the risk of skin cancer and early skin aging. -

Actinic Keratoses
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) Managing actinic keratoses Aim This pathway is designed to help GPs manage actinic keratoses effectively and reduce referrals to secondary care Background NICE estimates that over 23% of the UK population aged 60 and above have AKs Approximately 20% of AKs spontaneously resolve over a 1-year period. Some persist and a small number progress to squamous cell carcinoma. For a person with an average of 7.7 lesions, the probability of at least 1 lesion transforming to SCC in a 10-year period is ~10%. All patients diagnosed with AK in the community Except: Who? Immunosuppressed, especially those post transplant AKs induced by phototherapy e.g. PUVA for psoriasis Is there a past or family history of skin cancer or AK? Is the lesion painful? Diagnosis How quickly is it growing? Carefully examine the lesion & the REST of the patient’s skin Features of AK: www.dermnetnz.org/lesions/solar-keratoses Commonly develop on sun exposed sites in older people Forehead, face, back of hands, bald scalp of men, and ladies legs Early lesions may be red patches and produce pin-prick sensation. Later a sand paper roughness can be felt. Some become rough, raised and irregular, like stuck- on cornflakes Exclude painful, ulcerated or indurated lesions - these are signs of SCC and warrant a 2WW referral. If there’s a crust – take it off & look beneath! Cryotherapy or 5-fluorouracil cream (Efudix) are the usual starting points. Management 5-fluorouracil 5% cream (Efudix) is classified as ‘GREEN’ when prescribed in line with the managing actinic keratosis pathway.