Improving Anti-VEGF Drugs in the Vitreous by Eda Isil Altiok A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
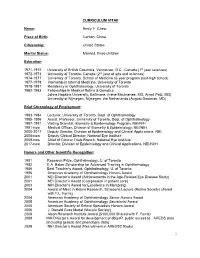
Emily Y. Chew, M.D
CURRICULUM VITAE Name: Emily Y. Chew Place of Birth: Canton, China Citizenship: United States Marital Status: Married, three children Education 1971-1972 University of British Columbia, Vancouver, B.C., Canada (1st year sciences) 1972-1973 University of Toronto, Canada (2nd year of arts and sciences) 1974-1977 University of Toronto, School of Medicine (6-year program post-high school) 1977-1978 Internship in Internal Medicine, University of Toronto 1978-1981 Residency in Ophthalmology, University of Toronto 1982-1983 Fellowships in Medical Retina & Genetics Johns Hopkins University, Baltimore, (Irene Maumenee, MD, Arnall Patz, MD) University of Nijmegen, Nijmegen, the Netherlands (August Deutman, MD) Brief Chronology of Employment: 1983-1984 Lecturer, University of Toronto, Dept. of Ophthalmology 1985-1986 Assist. Professor, University of Toronto, Dept. of Ophthalmology 1987-1991 Visiting Scientist, Biometry & Epidemiology Program, NEI/NIH 1991-now Medical Officer, Division of Biometry & Epidemiology, NEI/NIH 2000-2017 Deputy Director, Division of Epidemiology and Clinical Applications, NEI. 2008-now Deputy Clinical Director, National Eye Institute 2009-now Chief of Clinical Trials Branch, National Eye Institute 2017-now Director, Division of Epidemiology and Clinical Applications, NEI/NIIH Honors and Other Scientific Recognition: 1981 Research Prize, Ophthalmology, U. of Toronto 1982 E.A. Baker Scholarship for Advanced Training in Ophthalmology 1986 Best Teacher’s Award, Ophthalmology, U. of Toronto 1996 American Academy of Ophthalmology Honors Award 2001 NEI Director’s Award (Achievements in the Age-Related Eye Disease Study) 2001 NEI Director’s Award (Compassion in patient care) 2003 NIH Director’s Award for Excellence in Mentoring 2004 Award of Merit in Retina Research, Schepens Lecture (Retina Society) shared with F.L. -
Copyright and Use of This Thesis This Thesis Must Be Used in Accordance with the Provisions of the Copyright Act 1968
COPYRIGHT AND USE OF THIS THESIS This thesis must be used in accordance with the provisions of the Copyright Act 1968. Reproduction of material protected by copyright may be an infringement of copyright and copyright owners may be entitled to take legal action against persons who infringe their copyright. Section 51 (2) of the Copyright Act permits an authorized officer of a university library or archives to provide a copy (by communication or otherwise) of an unpublished thesis kept in the library or archives, to a person who satisfies the authorized officer that he or she requires the reproduction for the purposes of research or study. The Copyright Act grants the creator of a work a number of moral rights, specifically the right of attribution, the right against false attribution and the right of integrity. You may infringe the author’s moral rights if you: - fail to acknowledge the author of this thesis if you quote sections from the work - attribute this thesis to another author - subject this thesis to derogatory treatment which may prejudice the author’s reputation For further information contact the University’s Copyright Service. sydney.edu.au/copyright Clinical Studies into the Causes of Idiopathic Macular Telangiectasia Type 2: Sleep Apnoea and Macular Telangiectasia: The SAMTel Project Martin Lee Submitted to the Faculty of Medicine in fulfillment of the requirements of the degree Masters of Philosophy (Medicine) Department of Clinical Ophthalmology & Eye Health University of Sydney January 2015 Acknowledgements: To my family, especially parents, Anne and Russell, and siblings Alison and Frances, thank you for your unbridled support and endless encouragement to enable me to continue to pursue my educational interests. -

Macular Telangiectasia Type 2: Quantitative Analysis of a Novel
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Macular telangiectasia type 2: quantitative analysis of a novel phenotype and implications for the pathobiology of the disease Mali Okada, MBBS, MMed*; Catherine A Egan, FRANZCO*†; Tjebo FC Heeren, MD*‡; Adnan Tufail, MD, FRCOphth*; Marcus Fruttiger, PhD‡; Peter M Maloca, MD*§ * Moorfields Eye Hospital NHS Foundation Trust, London, UK † Department of Ophthalmology, University of Bonn, Bonn, Germany ‡ UCL Institute of Ophthalmology, London, United Kingdom § Department of Ophthalmology, University of Basel, Basel, Switzerland Short Title: Retinal microcystoid spaces in MacTel 2 Funding: Supported by the Lowy Medical Research Institute, Sydney, Australia as part of The Macular Telangiectasia Project (MO, CE). AT receives a proportion of funding from the National Institute for Health Research (NIHR) and Biomedical Research Centre at Moorfields Eye Hospital and the University College London Institute of Ophthalmology. The views expressed in the publication are those of the authors and not necessarily those of the Department of Health. Financial Disclosures: AT is on the advisory board for Heidelberg engineering (Heidelberg, Germany) and Optovue (Optovue Inc, USA). PM receives fees for lectures from Heidelberg engineering (Heidelberg, Germany) 1 Address for correspondence: Peter M Maloca, MD Moorfields Eye Hospital 162 City Road, London EC1V 2PD London, United Kingdom Tel: +44 20 72533411 Fax: +44 20 72534696 [email protected] Key words Macular telangiectasia type 2; spectral domain optical coherence tomography; volume rendering; microcystoid spaces; cystoid macular oedema; microcystic macular oedema; inner nuclear layer cysts; microcysts; microcavitations Summary Statement Retinal microcystoid spaces are a novel phenotype of Macular Telangiectasia (MacTel) type 2 on optical coherence tomography. -

Genetic Disruption of Serine Biosynthesis Is a Key Driver Of
bioRxiv preprint doi: https://doi.org/10.1101/2020.02.04.934356; this version posted February 5, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Genetic Disruption of Serine Biosynthesis is a Key 2 Driver of Macular Telangiectasia Type 2 Etiology and 3 Progression 4 5 RoBerto Bonelli,1,2 Brendan R E Ansell,1,2 Luca Lotta,3 Thomas Scerri,1,2 Traci E Clemons,4 Irene Leung,5 The 6 MacTel Consortium,6 Tunde Peto,7 Alan C Bird,8 Ferenc Sallo,9 Claudia LangenBerg,3 and Melanie Bahlo*1,2. 7 8 9 1 Department of Medical Biology, The University of Melbourne, 3052, Parkville, Victoria, Australia. 10 2 Population Health and Immunity Division, Walter and Eliza Hall Institute of Medical Research, 3052, Parkville, Victoria, 11 Australia. 12 3 MRC Epidemiology Unit, University of Cambridge, CB2 0SL, Cambridge, UK. 13 4 The EMMES Corporation, Rockville, 20850, Maryland, United States. 14 5 Department of Research and Development, Moorfields Eye Hospital NHS Foundation Trust, EC1V 2PD, London, United 15 Kingdom. 16 6 A list of members and affiliations is provided in Table S8. 17 7 Department of Ophthalmology, Queen’s University, Belfast, BT7 1NN, United Kingdom. 18 8 Inherited Eye Disease, Moorfields Eye Hospital NHS Foundation Trust, EC1V 2PD, London, United Kingdom. 19 9 Department of Ophthalmology, University of Lausanne, Hôpital Ophtalmique Jules-Gonin, Fondation Asile des aveugles, 20 Switzerland. -

Rotational Three-Dimensional OCTA
www.nature.com/scientificreports OPEN Rotational Three-dimensional OCTA: a Notable New Imaging Tool to Characterize Type 3 Macular Neovascularization Enrico Borrelli 1, Riccardo Sacconi 1, Gerd Klose2, Luis de Sisternes3, Francesco Bandello1 & Giuseppe Querques 1* This study explored whether rotational three-dimensional (3D) visualization of optical coherence tomography angiography (OCTA) volume data may yield valuable information regarding type 3 macular neovascularization (MNV). In this retrospective, cross-sectional study, we collected data from 15 eyes (13 patients) with treatment-naïve type 3 MNV in their post-nascent stage and age-related macular degeneration (AMD). Subjects were imaged with the SS-OCT system (PLEX Elite 9000, Carl Zeiss Meditec Inc., Dublin, CA, USA). The OCTA volume data were processed with a prototype volume projection removal algorithm and then analyzed using volumetric visualization techniques in order to obtain a 3D visualization of the region occupied by type 3 MNV. The two-dimensional and three-dimensional OCTA images were investigated. Mean ± SD age was 75.1 ± 7.4 years. BCVA was 0.42 ± 0.21 LogMAR in the study eyes. Considering the cohort of analyzed eyes, on rotational 3D OCTA images, a total of 35 neovascular lesions (vs 22 lesions detected on 2D OCTA images) rising from the deep vascular complex and variably spanning the outer retinal layers and eventually reaching the RPE/sub-RPE space were detected. Nine of 35 lesions had a saccular shape, while the remaining cases had a fliform shape. On rotational 3D OCTA images, these lesions were inclined on the three planes, instead of perpendicular to the RPE/Bruch’s membrane. -

Idiopathic Juxtafoveal Telangiectasis
RETINA HEALTH SERIES | Facts from the ASRS The Foundation American Society of Retina Specialists Committed to improving the quality of life of all people with retinal disease. Idiopathic Juxtafoveal Telangiectasis (pronounced tell an gee ACT te sis) (JFT), also known as SYMPTOMS perifoveal telangiectasis or mac-tel for macular telangiectasia, Patients with JFT may experience: is a condition in which abnormalities develop in blood vessels • Blurred vision at the center of the retina. This central part of the retina, • Metamorphopsia (pronounced called the fovea, is responsible for the sharp vision needed met a morf OP see ah)—a visual disturbance where straight lines for reading and recognizing faces (see retina diagram). appear wavy; parts of the central vision may also appear blank. • Difficulty reading • Visual field defects in one or both eyes The condition may be diagnosed before it causes any symptoms at all. WHAT IS THE RETINA? Early frames of the fluorescein angiogram show Later frames of the fluorescein angiogram show telangiectatic vessels in temporal macula. staining in the temporal macula. Photo courtesy Image courtesy of John Thompson, MD of John1 Thompson, MD 1 Causes and Risk Factors: There are several classifications of idiopathic (of an unknown cause) juxtafoveal telan- giectasis (JFT), but the most common one divides JFT into Types 1, 2 and 3. Types 1 and 3 are rare—the vast majority of patients with JFT have Type 2. JFT occurs when tiny abnormal blood THE RETINA is a thin layer of vessels (or telangiectasias—pronounced light-sensitive nerve tissue that lines tell an gec TAY shuhs) occur in the fovea. -

KAMAL KISHORE, MD 3602 Marquette Road Peru, IL 61354 Phone: (815)223-7400 E-Mail: [email protected]
KAMAL KISHORE, MD 3602 Marquette Road Peru, IL 61354 Phone: (815)223-7400 e-mail: [email protected] EDUCATION/EXPERIENCE Aug, 02 – Present President, Illinois Retina Institute, S.C. Aug, 99 – Jul, 02 Retina Surgeon, Bond Eye Associates, Peoria, IL Jul, 97 – Jun, 99 Fellow in Vitreo-Retinal Surgery Louisiana State University, New Orleans, LA Jul, 94 - Jun, 97 Residency in Ophthalmology UMDNJ-New Jersey Medical School, Newark, NJ Chief Resident: Jul, 96 - Jun, 97 Jan, 94 - Jun, 94 Research Fellowship in Ocular Oncology with Dr. Char UCSF, San Francisco, CA Jan, 92 - Dec, 93 SUNY at Stony Brook Program B Resident in Internal Medicine at Nassau County Med Ctr, NY Jan, 89 - Dec, 91 Senior Resident at Dr. R.P.Ctr for Ophth Sci., AIIMS, New Delhi, India Jan, 86 - Dec, 88 Residency in Ophthalmology Dr. R.P. Center for Oph Sci, AIIMS, New Delhi Jan, 85 - Dec, 85 Compulsory Internship (part of MBBS) Aug, 80 - Dec, 84 MBBS (Graduate Medical Education) All India Institute of Medical Sciences, New Delhi BOARD STATUS ABO Certified, October 1998 Recertified, January 1, 2009; January 1, 2019 LICENSURE California License #A 053457, dated 8/24/94, inactive IL Physician and Surgeon License #036-100858, 8/3/99 1 SCHOLARSHIPS AND AWARDS Jul, 77 - Jun, 79 Merit Scholarship Jul, 79 - Dec, 88 National Talent Scholarship Year 1982 Medal for best medical student in Microbiology Merit Award for standing first in Pathology Year 1988 Medal for best graduate in Ophthalmology Aug, 1992 House Officer of the month, Nassau County Med Ctr Jun, 1997 Alfonse Cinotti Award for best graduate at UMDNJ August, 2014 Technovisionary Award, Film Festival, ASRS, San Diego, CA July, 2015 Honor Award, American Society of Retina Specialists, (ASRS) August, 2017 Senior Honor Award, ASRS, Boston, MA PUBLICATIONS Book Chapters Kishore K. -

Ucla Stein Eye Institute Vision-Science Campus
SPRING 2020 VOLUME 38 NUMBER 1 UCLA STEIN EYE INSTITUTE EYEVISION-SCIENCE CAMPUS LETTER FROM THE CHAIR To have 20/20 vision is to see clearly, and for the UCLA Department of Ophthalmology, the year 2020 heralds in both a new decade and a focus on our mission to preserve sight and end avoidable blindness. Research is core to this aim, and in this issue of EYE Magazine, we highlight basic scientists in the UCLA Stein Eye Institute’s Vision Science Division who are studying the fundamental mechanisms of visual function and using that knowledge to define, identify, and ultimately cure eye disease. Clinical research trials are also underway at Stein Eye and the Doheny Eye Centers UCLA, and include the first in-human trial of autologous cultivated limbal cell therapy to treat limbal stem cell deficiency, a blinding corneal disease; evaluation of an investiga- tional medication to treat graft-versus-host disease, a potentially EYE MAGAZINE is a publication of the serious complication after transplant procedures; comparison of UCLA Stein Eye Institute drug-delivery systems to determine which is most effective for DIRECTOR treatment of diabetic macular edema; and analysis of regenerative Bartly J. Mondino, MD strategies for treatment of age-related macular degeneration. MANAGING EDITOR UCLA Department of Ophthalmology award-winning researchers Tina-Marie Gauthier conduct investigations of depth and magnitude, and the Depart- c/o Stein Eye Institute 100 Stein Plaza, UCLA ment has taken a central role in transforming vision science as a Los Angeles, California 90095–7000 powerful platform for discovery. I thank our faculty and staff for their [email protected] sight-saving endeavors, and I thank you, our donors and friends, for PUBLICATION COMMITTEE supporting our scientific explorations. -

Engelbert CV
Michael Engelbert, MD PhD 460 Park Avenue, 5th Floor New York, NY 10022 Phone: (212) 861-9797 Fax: (212) 628-0698 Education Ph.D. Oklahoma University Health Sciences Center, Oklahoma, USA – Microbiology and Immunology Thesis presented at “Hot Topics in Microbiology,” ARVO 2004 M.D. Ludwig-Maximilians-Universität, München, Germany – Medical School, magna cum laude Last Year Electives and Final Board Exam, ECFMG certification Technische Universität, München, Germany – Medical School Clinical Semesters Student Government Representative 2001 – 2002 Universität RegensBurg, Germany – Premedicine Baccalaureate Hans-Carossa Preparatory School, Landshut, Germany, amongst top 10 graduates Postgraduate Training • ColumBia University, Dr. Stanley Chang 2008 – 2010 Vitreous Retina Macula Consultants oF New York, Dr. Lawrence A. Yannuzzi New York Eye and Ear InFirmary, Dr. Richard Rosen Clinical and Surgical Fellow in Diseases of the Retina and Vitreous • ColumBia University, Edward S. Harkness Eye Institute 2005 – 2008 Resident and Chief Resident in Ophthalmology • GriFFin Hospital / Yale University School oF Medicine, Vincent A. De 2004 – 2005 Luca Intern of the Year Award Medical Internship Current Employment • Vitreous Retina Macula Consultants oF New York, Associate 2010 – Present • New York University, Research Assistant Professor 2010 – Present • Bellevue Hospital, Assistant Attending 2010 – Present • New York Eye and Ear InFirmary; Manhattan Eye, Ear & Throat 2010 – Present Hospital, Attending Physician Michael Engelbert, MD PhD Page 2 Honors and Awards • Permanent Resident Status awarded as physician-scientist at top of 2009 his particular field • Best Poster Presentation, Euretina Winter Meeting – Rome, Italy 2014 • Macula Foundation Travel Awards o Euretina 2008, 2010 o ARVO 2009, 2011 • Circle of Excellence, Arnold P. Gold Foundation’s Humanism and 2008 Excellence in Teaching Award • AAO Advocacy Day, travel award by New York State 2007 Ophthalmological Society • Travel Award to attend Heed Foundation Residency Retreat 2006 • Vincent A. -

Idiopathic Peripheral Retinal Telangiectasia in Adults: a Case Series and Literature Review
Journal of Clinical Medicine Article Idiopathic Peripheral Retinal Telangiectasia in Adults: A Case Series and Literature Review Maciej Gaw˛ecki Dobry Wzrok Ophthalmological Clinic, 80-822 Gdansk, Poland; [email protected]; Tel.: +48-501-788-654 Abstract: Idiopathic peripheral retinal telangiectasia (IPT), often termed as Coats disease, can present in a milder form with the onset in adulthood. The goal of this case series study and literature review was to describe and classify different presenting forms and treatment of this entity and to review contemporary methods of its management. Six cases of adult onset IPT were described with the following phenotypes based on fundus ophthalmoscopy, fluorescein angiography, and optical coherence tomography findings: IPT without exudates or foveal involvement, IPT with peripheral exudates without foveal involvement, IPT with peripheral exudates and cystoid macular edema, and IPT with peripheral and macular hard exudates. Treatments applied in this series included observation, laser photocoagulation, and anti-vascular endothelial growth factor (VEGF) treatment with variable outcomes depending upon the extent of IPT, the aggressiveness of laser treatment, and the stringency of follow-up. The accompanying literature review suggests that ablative therapies, especially laser photocoagulation, remain the most effective treatment option in adult-onset IPT, with anti-VEGF therapy serving as an adjuvant procedure. Close follow-up is necessary to achieve and maintain reasonable good visual and morphological results. Keywords: Coats disease; peripheral retinal telangiectasia; laser photocoagulation; anti-VEGF treatment Citation: Gaw˛ecki,M. Idiopathic Peripheral Retinal Telangiectasia in 1. Introduction Adults: A Case Series and Literature Review. J. Clin. Med. 2021, 10, 1767. Idiopathic peripheral retinal telangiectasia (IPT), usually referred to as Coats disease, https://doi.org/10.3390/jcm10081767 has been well-described in the medical literature since its discovery in 1908 [1]. -

Art for Macular Holes: Outcomes of the World Study
s DIVERSITY AND INCLUSION Retina Around the World Experts share the latest research and preferred techniques for macular holes, nonexudative macular neovascularization, myopic traction maculopathy, and more. BY JUDY E. KIM, MD; LIHTEH WU, MD; TAMER H. MAHMOUD, MD, PHD; GIUSEPPE QUERQUES, MD, PHD; KAZUAKI KADONOSONO, MD, PHD; GEMMY CHEUNG, MD; PAUL S. BERNSTEIN, MD, PHD; AND JOSE A. ROCA, MD Despite the pandemic, our colleagues around the world continue to explore ways to improve the diagnosis and management of various retinal conditions. Because of COVID-19, perhaps the world has become smaller, as we share information in virtual settings. During the virtual 2020 annual meeting of the AAO, we gathered an international group of experts to share their knowledge and learn the latest research findings from various corners of the world. We are excited to share with you their summaries of the research they presented. – Judy E. Kim, MD, and Lihteh Wu, MD neurosensory layers (ANL). When the graft is first placed, ART FOR MACULAR HOLES: vertical lines appear on OCT between the graft and sur- OUTCOMES OF THE WORLD STUDY rounding macular tissue. Within weeks, these lines gradually fade, details of the graft layers can be detected, and they By Tamer H. Mahmoud, MD, PhD align with similar layers in the surrounding host macular A multicenter international interventional tissue (ie, plexiform to plexiform, nuclear to nuclear, etc.). study with 33 participating surgeons looked This could suggest that the macular tissue recognizes the at 130 eyes that underwent autologous reti- peripheral retinal tissue and may be trying to connect to nal transplant (ART) for repair of macular corresponding layers, leading to integration of the transplant holes (MH) and MH retinal detachment (MHRD) to deter- and, thus, better visual outcomes. -

Characteristics and Quantification of Vascular Changes in Macular
Downloaded from http://bjo.bmj.com/ on November 15, 2016 - Published by group.bmj.com Clinical science Characteristics and quantification of vascular changes in macular telangiectasia type 2 on optical coherence tomography angiography Lavanya Chidambara,1 Santosh G K Gadde,1 Naresh Kumar Yadav,1 Chaitra Jayadev,1 Devanshi Bhanushali,1 Abhishek M Appaji,2 Mukunda Akkali,1 Aruj Khurana,1 Rohit Shetty1 1Narayana Nethralaya Eye ABSTRACT MacTel type 2 (MacTel 2) is a bilateral disease Hospital, Bangalore, India Background Macular telangiectasia type 2 (MacTel 2) characterised by changes in the capillary network 2BMS College of Engineering, Bangalore, India is a bilateral idiopathic, rare neurodegenerative disease and neurosensory atrophy. Slit lamp biomicroscopy with alterations in the macular capillary network leading reveals reduced retinal transparency, crystalline Corresponding to to vision loss and is the most common of three subtypes. deposits, ectatic capillaries and blunted venules.4 Dr Santosh G K Gadde, Optical coherence tomography angiography (OCTA) is a Complications of this disorder include proliferation Department of Vitreo-retina, non-invasive imaging modality which helps understand of pigment plaques, photoreceptor loss, foveal Narayana Nethralaya, #121, Chord Road, 1st ‘R’ Block, the complex pathological changes, and images the blood atrophy and retinal neovascularisation that may 5 Rajaji Nagar, Bangalore, vessels across different layers based on their flow result in visual loss. Karnataka 560010, India; characteristics. Stereoscopic colour and red-free fundus photog- [email protected] Methods A cross-sectional study was conducted on raphy and fundus fluorescein angiography (FA) Received 7 October 2015 56 eyes of Asian Indian eyes of 28 consecutive patients have been previously used to describe the disease.