Establishment of the Mayfly Cloeon Dipterum As a New Model System to Investigate Insect Evolution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

No Evidence for Immune-Gene Specific Signals of Selection in Termites
bioRxiv preprint doi: https://doi.org/10.1101/783738; this version posted September 26, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. No evidence for immune-gene specific signals of selection in termites 1Running title: Selection on termite immune genes 2Karen Meusemann1,2, Judith Korb¹, Maximilian Schughart¹, Fabian Staubach1* 31Evolutionary Biology & Ecology, Biology I (Animal Zoology), University of Freiburg, Freiburg 4(Brsg.), Germany 52Australian National Insect Collection, CSIRO, Acton, Canberra, ACT, Australia 6* Correspondence: 7Fabian Staubach [email protected] 9 10Life Science Identifiers (as available Zoobank) 11Ephemera danica: 12urn:lsid:zoobank.org:act:06633F75-4809-4BB3-BDCB-6270795368D5 13Coptotermes sp. 14urn:lsid:zoobank.org:pub:D6724B7F-F27A-47DC-A4FC-12859ECA0C71 15Blattella germanica: 16rn:lsid:zoobank.org:pub:1EA126BA-E9D2-4AA6-8202-26BA5B09B8AD 17Locusta migratoria 18urn:lsid:zoobank.org:pub:D792A09E-844A-412A-BFCA-5293F8388F8C 19Periplaneta americana (Blatta americana): 20urn:lsid:zoobank.org:act:95113A55-4C6D-4DC7-A0E5-620BACADFFE5 21Apis mellifera: 22urn:lsid:zoobank.org:act:9082C709-6347-4768-A0DC-27DC44400CB2 23Bombyx mori (Phalæna (Bombyx) mori) 24urn:lsid:zoobank.org:act:215466E3-E77F-46E9-8097-372837D7A375 25Drosophila melanogaster: 26urn:lsid:zoobank.org:act:5B39F0AA-270D-4AA8-B9A3-C36A3A265910 27 28Keywords: immunity, social insects, termites, selection, comparative genomics 29Abstract 30It has been hypothesized that selection pressure from pathogens plays an important role in shaping 31social evolution. Social behaviour, in particular brood care, is associated with pathogen pressure in 32wood-dwelling “lower” termites. Yet, generally pathogen pressure is low in wood-dwelling termite 33species that never leave the nest except for the mating flight. -

United States National Museum Bulletin 276
,*f»W*»"*^W»i;|. SMITHSONIAN INSTITUTION MUSEUM O F NATURAL HISTORY UNITED STATES NATIONAL MUSEUM BULLETIN 276 A Revision of the Genus Malacosoma Hlibner in North America (Lepidoptera: Lasiocampidae): Systematics, Biology, Immatures, and Parasites FREDERICK W. STEHR and EDWIN F. COOK SMITHSONIAN INSTITUTION PRESS CITY OF WASHINGTON 1968 PUBLICATIONS OF THE UNITED STATES NATIONAL MUSEUM The scientific publications of the United States National Museum include two series. Proceedings of the United States National Museum and United States National Museum Bulletin. In these series are published original articles and monographs dealing with the collections and work of the Museum and setting forth newly acquired facts in the field of anthropology, biology, geology, history, and technology. Copies of each publication are distributed to libraries and scientific organizations and to specialists and others interested in the various subjects. The Proceedings, begun in 1878, are intended for the publication, in separate form, of shorter papers. These are gathered in volumes, octavo in size, with the publication date of each paper recorded in the table of contents of the volume. In the Bulletin series, the first of which was issued in 1875, appear longer, separate publications consisting of monographs (occasionally in several parts) and volumes in which are collected works on related subjects. Bulletins are either octavo or quarto in size, depending on the needs of the presentation. Since 1902, papers relating to the botanical collections of the Museum have been published in the Bulletin series under the heading Contributions from the United States National Herbarium. This work forms number 276 of the Bulletin series. -
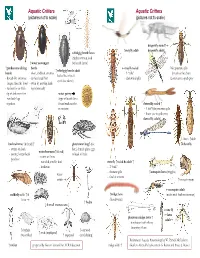
Aquatic Critters Aquatic Critters (Pictures Not to Scale) (Pictures Not to Scale)
Aquatic Critters Aquatic Critters (pictures not to scale) (pictures not to scale) dragonfly naiad↑ ↑ mayfly adult dragonfly adult↓ whirligig beetle larva (fairly common look ↑ water scavenger for beetle larvae) ↑ predaceous diving beetle mayfly naiad No apparent gills ↑ whirligig beetle adult beetle - short, clubbed antenna - 3 “tails” (breathes thru butt) - looks like it has 4 - thread-like antennae - surface head first - abdominal gills Lower jaw to grab prey eyes! (see above) longer than the head - swim by moving hind - surface for air with legs alternately tip of abdomen first water penny -row bklback legs (fbll(type of beetle larva together found under rocks damselfly naiad ↑ in streams - 3 leaf’-like posterior gills - lower jaw to grab prey damselfly adult↓ ←larva ↑adult backswimmer (& head) ↑ giant water bug↑ (toe dobsonfly - swims on back biter) female glues eggs water boatman↑(&head) - pointy, longer beak to back of male - swims on front -predator - rounded, smaller beak stonefly ↑naiad & adult ↑ -herbivore - 2 “tails” - thoracic gills ↑mosquito larva (wiggler) water - find in streams strider ↑mosquito pupa mosquito adult caddisfly adult ↑ & ↑midge larva (males with feather antennae) larva (bloodworm) ↑ hydra ↓ 4 small crustaceans ↓ crane fly ←larva phantom midge larva ↑ adult→ - translucent with silvery bflbuoyancy floats ↑ daphnia ↑ ostracod ↑ scud (amphipod) (water flea) ↑ copepod (seed shrimp) References: Aquatic Entomology by W. Patrick McCafferty ↑ rotifer prepared by Gwen Heistand for ACR Education midge adult ↑ Guide to Microlife by Kenneth G. Rainis and Bruce J. Russel 28 How do Aquatic Critters Get Their Air? Creeks are a lotic (flowing) systems as opposed to lentic (standing, i.e, pond) system. Look for … BREATHING IN AN AQUATIC ENVIRONMENT 1. -

The Mayfly Newsletter: Vol
Volume 20 | Issue 2 Article 1 1-9-2018 The aM yfly Newsletter Donna J. Giberson The Permanent Committee of the International Conferences on Ephemeroptera, [email protected] Follow this and additional works at: https://dc.swosu.edu/mayfly Part of the Biology Commons, Entomology Commons, Systems Biology Commons, and the Zoology Commons Recommended Citation Giberson, Donna J. (2018) "The aM yfly eN wsletter," The Mayfly Newsletter: Vol. 20 : Iss. 2 , Article 1. Available at: https://dc.swosu.edu/mayfly/vol20/iss2/1 This Article is brought to you for free and open access by the Newsletters at SWOSU Digital Commons. It has been accepted for inclusion in The Mayfly eN wsletter by an authorized editor of SWOSU Digital Commons. An ADA compliant document is available upon request. For more information, please contact [email protected]. The Mayfly Newsletter Vol. 20(2) Winter 2017 The Mayfly Newsletter is the official newsletter of the Permanent Committee of the International Conferences on Ephemeroptera In this issue Project Updates: Development of new phylo- Project Updates genetic markers..................1 A new study of Ephemeroptera Development of new phylogenetic markers to uncover island in North West Algeria...........3 colonization histories by mayflies Sereina Rutschmann1, Harald Detering1 & Michael T. Monaghan2,3 Quest for a western mayfly to culture...............................4 1Department of Biochemistry, Genetics and Immunology, University of Vigo, Spain 2Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Berlin, Germany 3 Joint International Conf. Berlin Center for Genomics in Biodiversity Research, Berlin, Germany Items for the silent auction at Email: [email protected]; [email protected]; [email protected] the Aracruz meeting (to sup- port the scholarship fund).....6 The diversification of evolutionary young species (<20 million years) is often poorly under- stood because standard molecular markers may not accurately reconstruct their evolutionary How to donate to the histories. -

CHAPTER 4: EPHEMEROPTERA (Mayflies)
Guide to Aquatic Invertebrate Families of Mongolia | 2009 CHAPTER 4 EPHEMEROPTERA (Mayflies) EPHEMEROPTERA Draft June 17, 2009 Chapter 4 | EPHEMEROPTERA 45 Guide to Aquatic Invertebrate Families of Mongolia | 2009 ORDER EPHEMEROPTERA Mayflies 4 Mayfly larvae are found in a variety of locations including lakes, wetlands, streams, and rivers, but they are most common and diverse in lotic habitats. They are common and abundant in stream riffles and pools, at lake margins and in some cases lake bottoms. All mayfly larvae are aquatic with terrestrial adults. In most mayfly species the adult only lives for 1-2 days. Consequently, the majority of a mayfly’s life is spent in the water as a larva. The adult lifespan is so short there is no need for the insect to feed and therefore the adult does not possess functional mouthparts. Mayflies are often an indicator of good water quality because most mayflies are relatively intolerant of pollution. Mayflies are also an important food source for fish. Ephemeroptera Morphology Most mayflies have three caudal filaments (tails) (Figure 4.1) although in some taxa the terminal filament (middle tail) is greatly reduced and there appear to be only two caudal filaments (only one genus actually lacks the terminal filament). Mayflies have gills on the dorsal surface of the abdomen (Figure 4.1), but the number and shape of these gills vary widely between taxa. All mayflies possess only one tarsal claw at the end of each leg (Figure 4.1). Characters such as gill shape, gill position, and tarsal claw shape are used to separate different mayfly families. -

CONTRIBUTIONS to a REVISED SPECIES CONSPECT of the EPHEMEROPTERA FAUNA from ROMANIA (Mayfliesyst)
Studii şi Cercetări Mai 2014 Biologie 23/2 20-30 Universitatea”Vasile Alecsandri” din Bacău CONTRIBUTIONS TO A REVISED SPECIES CONSPECT OF THE EPHEMEROPTERA FAUNA FROM ROMANIA (mayfliesyst) Florian S. Prisecaru, Ionel Tabacaru, Maria Prisecaru, Ionuţ Stoica, Maria Călin Key words: Ephemeroptetera, systematic classification, new species, Romania. INTRODUCTION wrote the chapter Order Ephemeroptera (2007, pp.235-236) and mentioned 108 species in the list of In the volume „Lista faunistică a României Ephemeroptera from our country, indicating the (specii terestre şi de apă dulce) [List of Romanian authors of their citation. It is the first time since the fauna (terrestrial and freshwater species)], editor-in- publication of a fauna volume (Bogoescu, 1958) that chief Anna Oana Moldovan from "Emil Racovita" such a list has been made public. Here is this list Institute of Speleology, Cluj-Napoca, Milca Petrovici followed by our observations. 0rder EPHEMEROPTERA Superfamily BAETISCOIDEA Family PROSOPISTOMATIDAE Genus Species Author, year 1. Prosopistoma pennigerum Mueller, 1785 Superfamily BAETOIDEA Family AMETROPODIDAE 2. Ametropus fragilis Albarda, 1878 Family BAETIDAE 3. Acentrella hyaloptera Bogoescu, 1951 4. Acentrella inexpectata Tschenova, 1928 5. Acentrella sinaica Bogoescu, 1931 6. Baetis alpinus Pictet, 1843 7. Baetis buceratus Eaton, 1870 8. Baetis fuscatus Linnaeus, 1761 9. Baetis gracilis Bogoescu and Tabacaru, 1957 10. Baetis lutheri Eaton, 1885 11. Baetis melanonyx Bogoescu, 1933 12. Baetis muticus Bürmeister, 1839 13. Baetis niger Linnaeus, 1761 14. Baetis rhodani Pictet, 1843 15. Baetis scambus Eaton, 1870 16. Baetis tenax Eaton, 1870 17. Baetis tricolor Tschenova,1828 18. Baetis vernus Curtis, 1864 19. Centroptilum luteolum Müller, 1775 20. Cloeon dipterum Linné, 1761 21. -

Yellow Mayfly (Potamanthus Luteus )
SPECIES MANAGEMENT SHEET Yellow mayfly (Potamanthus luteus ) The Yellow mayfly is one of Britain’s rarest mayflies. The nymphs or larvae of this mayfly typically live in silt trapped amongst stones on the riverbed in pools and margins and grow to between 15 and 17mm. They are streamlined with seven pairs of thick feathery gills that are held outwards from their sides. The adults have three tails and large hindwings. The body is a dull yellowish-orange with a distinctive broad yellowish brown stripe along the back. The wings are yellow and the cross-veins are a dark reddish colour. Due to its rarity and decline in numbers this insect has been made a Priority Species on the UK Biodiversity Action Plan (BAP). Life cycle There is one generation of this mayfly a year which overwinters as larvae. The adult mayflies are short lived and emerge between May and late October (with peak emergence in July). They will typically emerge at dusk and usually from the surface of the water, although they may also emerge by climbing up stones or plant stems partially or entirely out of water. Distribution map This mayfly is historically a rare species with populations in the River Wye and Usk, Herefordshire. The most recent surveys show a dramatic decline in the River Wye population and have failed to find this species in the River Usk. A small population has however recently been found in the River Teme in Worcestershire. Habitat In the Herefordshire streams where this species has been found, the larvae have been found under loose stones, preferring mobile sections of shingle or a mixture of larger stones with loose shingle such as those found downstream of bridges or at the confluence of tributaries. -

TB142: Mayflies of Maine: an Annotated Faunal List
The University of Maine DigitalCommons@UMaine Technical Bulletins Maine Agricultural and Forest Experiment Station 4-1-1991 TB142: Mayflies of aine:M An Annotated Faunal List Steven K. Burian K. Elizabeth Gibbs Follow this and additional works at: https://digitalcommons.library.umaine.edu/aes_techbulletin Part of the Entomology Commons Recommended Citation Burian, S.K., and K.E. Gibbs. 1991. Mayflies of Maine: An annotated faunal list. Maine Agricultural Experiment Station Technical Bulletin 142. This Article is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Technical Bulletins by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. ISSN 0734-9556 Mayflies of Maine: An Annotated Faunal List Steven K. Burian and K. Elizabeth Gibbs Technical Bulletin 142 April 1991 MAINE AGRICULTURAL EXPERIMENT STATION Mayflies of Maine: An Annotated Faunal List Steven K. Burian Assistant Professor Department of Biology, Southern Connecticut State University New Haven, CT 06515 and K. Elizabeth Gibbs Associate Professor Department of Entomology University of Maine Orono, Maine 04469 ACKNOWLEDGEMENTS Financial support for this project was provided by the State of Maine Departments of Environmental Protection, and Inland Fisheries and Wildlife; a University of Maine New England, Atlantic Provinces, and Quebec Fellow ship to S. K. Burian; and the Maine Agricultural Experiment Station. Dr. William L. Peters and Jan Peters, Florida A & M University, pro vided support and advice throughout the project and we especially appreci ated the opportunity for S.K. Burian to work in their laboratory and stay in their home in Tallahassee, Florida. -

A Comparison of Aquatic Invertebrate Assemblages Collected from the Green River in Dinosaur National Monument in 1962 and 2001
A Comparison of Aquatic Invertebrate Assemblages Collected from the Green River in Dinosaur National Monument in 1962 and 2001 Final Report for United States Department of the Interior National Park Service Dinosaur National Monument 4545 East Highway 40 Dinosaur, Colorado 81610-9724 Report Prepared by: Dr. Mark Vinson, Ph.D. & Ms. Erin Thompson National Aquatic Monitoring Center Department o f Fisheries and Wildlife Utah State University Logan, Utah 84322-5210 www.usu.edu/buglab 22 January 2002 i Foreword The work described in this report was conducted by personnel of the National Aquatic Monitoring Center, Utah State University, Logan, Utah. Mr. J. Matt Tagg aided in the identification of the aquatic invertebrates. Ms. Leslie Ogden provided computer assistance. Several people at Dinosaur National Monument helped us immensely with various project details. Steve Petersburg, Dana Dilsaver, and Dennis Ditmanson provided us with our research permit. Ann Elder helped with sample archiving. Christy Wright scheduled our trip and provided us with our river permit. We thank them all for all their help and good spirit. We also thank Mr. Walter Kittams (National Park Service, Regional Office, Omaha Nebraska) and Mr. Earl M. Semingsen (Superintendent, Dinosaur National Monument) for funding the study and the members of the 1962 University of Utah expedition: Dr. Angus M. Woodbury, Dr. Stephen Durrant, Mr. Delbert Argyle, Mr. Douglas Anderson, and Dr. Seville Flowers for their foresight to conduct the original study nearly 40 years ago. The concept and value of long-term ecological data is often bantered about, but its value is never more apparent then when we conduct studies like that presented here. -

Browntail Moth and the Big Itch: Public Health Implications and Management of an Invasive Species
Browntail Moth and the Big Itch: Public Health Implications and Management of an Invasive Species July 22, 2021 Jill H. Colvin, MD, FAAD MDFMR Dermatology photo credits: Jon Karnes, MD Thomas E. Klepach, PhD Assistant Professor of Biology, Colby College Ward 3 City Councilor, Waterville, Maine Financial disclosure statement Jill H Colvin MD and Thomas E. Klepach, PhD do not have a financial interest/arrangement or affiliation with any organizations that could be perceived as a conflict of interest in the context of this presentation. OBJECTIVES • Identify: • Impacts of browntail moth (BTM) infestation on humans and the environment • Review: • Presentation and treatment of BTM dermatoses • Biology of the BTM • Population trends of the BTM • BTM mitigation strategies JUNE 2021, AUGUSTA, MAINE PERVASIVE! COMPLICATIONS OF INVASIVE BROWNTAIL MOTH SOCIAL MEDIA RADIO ARBORISTS PHARMACY NEWSPAPERS FRIENDS COLLEAGUES HEALTH CARE FAMILY TEXTS TOURISM PATIENTS MAINE.GOV SELF REALTY PHONE CALLS ACTIVISM TREES SOCIAL MEDIA IMPACT: TOURISM, REAL ESTATE, DAILY LIFE Maine.gov Maine.gov Photo credit: Jon Karnes, MD Photo credit: Robert Kenney, DO BTM: Risk to Human Health Mediated by: Toxic hairs on caterpillars, female moths, environment Direct contact Airborne Damage skin, eye, airway in multiple ways Mechanical: Barbs on hair Chemical: toxin Irritant Allergen One report of Death – 1914: ”severe internal poisoning caused by inhaling the hairs” Definitions Lepidopterism is the term for cutaneous and systemic reactions that result from contact with larvae (ie, caterpillars) or adult forms of moths and butterflies (order, Lepidoptera). Erucism (from Latin "eruca," caterpillar) is also used to refer to reactions from contact with caterpillars. photo credit: Jon Karnes, MD “The BTM, its caterpillar and their rash” Clinical and Experimental Dermatology (1979), Cicely P. -

Appendix Page
NORTH CAROLINA DEPARTMENT OF ENVIRONMENT AND NATURAL RESOURCES Division of Water Quality Environmental Sciences Section August 2004 This page was intentionally left blank NCDENR, Division of Water Quality Basinwide Assessment Report – Cape Fear River Basin - August 2004 1 TABLE OF CONTENTS Page LIST OF APPENDICIES ........................................................................................................................ 5 LIST OF TABLES................................................................................................................................... 7 LIST OF FIGURES .............................................................................................................................. 11 OVERVIEW OF THE WATER QUALITY OF THE CAPE FEAR RIVER BASIN.....................................17 EXECUTIVE SUMMARIES BY PROGRAM AREA.................................................................................27 FISHERIES ...................................................................................................................................... 27 BENTHIC MACROINVERTEBRATES............................................................................................. 30 LAKE ASSESSMENT....................................................................................................................... 32 PHYTOPLANKTON MONITORING................................................................................................. 33 AMBIENT MONITORING................................................................................................................ -

Distribution of Mayfly Species in North America List Compiled from Randolph, Robert Patrick
Page 1 of 19 Distribution of mayfly species in North America List compiled from Randolph, Robert Patrick. 2002. Atlas and biogeographic review of the North American mayflies (Ephemeroptera). PhD Dissertation, Department of Entomology, Purdue University. 514 pages and information presented at Xerces Mayfly Festival, Moscow, Idaho June, 9-12 2005 Acanthametropodidae Ameletus ludens Needham Acanthametropus pecatonica (Burks) Canada—ON,NS,PQ. USA—IL,GA,SC,WI. USA—CT,IN,KY,ME,MO,NY,OH,PA,WV. Ameletus majusculus Zloty Analetris eximia Edmunds Canada—AB. Canada—AB ,SA. USA—MT,OR,WA. USA—UT,WY. Ameletus minimus Zloty & Harper USA—OR. Ameletidae Ameletus oregonenesis McDunnough Ameletus amador Mayo Canada—AB ,BC,SA. Canada—AB. USA—ID,MT,OR,UT. USA—CA,OR. Ameletus pritchardi Zloty Ameletus andersoni Mayo Canada—AB,BC. USA—OR,WA. Ameletus quadratus Zloty & Harper Ameletus bellulus Zloty USA—OR. Canada—AB. Ameletus shepherdi Traver USA—MT. Canada—BC. Ameletus browni McDunnough USA—CA,MT,OR. Canada—PQ Ameletus similior McDunnough USA—ME,PA,VT. Canada—AB,BC. Ameletus celer McDunnough USA—CO,ID,MT,OR,UT Canada—AB ,BC. Ameletus sparsatus McDunnough USA—CO,ID,MT,UT Canada—AB,BC,NWT. Ameletus cooki McDunnough USA—AZ,CO,ID,MT,NM,OR Canada—AB,BC. Ameletus subnotatus Eaton USA—CO,ID,MT,OR,WA. Canada—AB,BC,MB,NB,NF,ON,PQ. Ameletus cryptostimulus Carle USA—CO,UT,WY. USA—NC,NY,PA,SC,TN,VA,VT,WV. Ameletus suffusus McDunnough Ameletus dissitus Eaton Canada—AB,BC. USA—CA,OR. USA—ID,OR. Ameletus doddsianus Zloty Ameletus tarteri Burrows USA—AZ,CO,NM,NV,UT.