Nail Psoriasis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proper Preop Makes for Easier Toenail Surgery
April 15, 2007 • www.familypracticenews.com Skin Disorders 25 Proper Preop Makes for Easier Toenail Surgery BY JEFF EVANS sia using a digital block or a distal approach to take ef- Senior Writer fect. Premedication with NSAIDs, codeine, or dextro- propoxyphene also may be appropriate, he said. WASHINGTON — Proper early management of in- To cut away the offending section of nail, an English grown toenails may help to decrease the risk of recur- anvil nail splitter is inserted under the nail plate and the rence whether or not surgery is necessary, Dr. C. Ralph cut is made all the way to the proximal nail fold. The hy- Daniel III said at the annual meeting of the American pertrophic, granulated tissue should be cut away as well. Academy of Dermatology. Many ingrown toenails are recurrent, so Dr. Daniel per- “An ingrown nail is primarily acting as a foreign-body forms a chemical matricectomy in nearly all patients after reaction. That rigid spicule penetrates soft surrounding tis- making sure that the surgical field is dry and bloodless. sue” and produces swelling, granulation tissue, and some- The proximal nail fold can be flared back to expose more times a secondary infection, said Dr. Daniel of the de- of the proximal matrix if necessary. Dr. Daniel inserts a Cal- partments of dermatology at the University of Mississippi, giswab coated with 88% phenol or 10% sodium hydroxide Jackson, and the University of Alabama, Birmingham. and applies the chemical for 30 seconds to the portion of For the early management of stage I ingrown toenails the nail matrix that needs to be destroyed. -

Sole Training® with Stacey Lei Krauss
Sole Training® with Stacey Lei Krauss Sole Training is a foot fitness program based on two sequences. The self –massage sequence is restorative and therapeutic; compare it to a yoga class (for your feet). The standing sequence promotes strength, endurance, flexibility and coordination; compare it to a boot-camp workout (for your feet). These exercises work; we’ve been doing them for over a decade. *The Sole Training® video download is available at willPowerMethod.com What is foot fitness? Building muscular strength, endurance, flexibility and neuro-muscular awareness in the feet and ankles. What are the benefits of foot fitness? According to Vibram FiveFingers®, exercising while barefoot, or wearing minimal shoes provide the following benefits: 1. Strengthens Muscles in the Feet and Lower Legs Wearing minimal shoes, or training barefoot will stimulate and strengthen muscles in the feet and lower legs, improving general foot health and reducing the risk of injury. 2. Improves Range of Motion in Ankles, Feet and Toes No longer 'cast' in a traditional, structured shoe, the foot and toes move more naturally. 3. Stimulates Neural Function Important to Balance and Agility When barefoot or wearing minimal shoes, thousands of neurological receptors in the feet send valuable information to the brain, improving balance and agility. 4. Eliminate Heel Lift to Align the Spine and Improve Posture By lowering the heel, your bodyweight becomes evenly distributed across the footbed, promoting proper posture and spinal alignment. 5. Allow the Foot and Body to Move Naturally Which just FEELS GOOD. [email protected] Sole Training® 1 Sole Training® with Stacey Lei Krauss Sole Training® Massage Sequence preparation: mats, blankets, blocks, towels, foot lotion time: 3-10 minutes when: prior to any workout, after any workout, before bed or upon waking EXERCISE EXECUTION FUNCTION Use your fingers to lengthen your toes: LOCALLY: Circulation, Toe flexibility and mobility leading TOE • Long stretch (3 joints except Big Toe) to enhanced balance. -

Fungal Infections from Human and Animal Contact
Journal of Patient-Centered Research and Reviews Volume 4 Issue 2 Article 4 4-25-2017 Fungal Infections From Human and Animal Contact Dennis J. Baumgardner Follow this and additional works at: https://aurora.org/jpcrr Part of the Bacterial Infections and Mycoses Commons, Infectious Disease Commons, and the Skin and Connective Tissue Diseases Commons Recommended Citation Baumgardner DJ. Fungal infections from human and animal contact. J Patient Cent Res Rev. 2017;4:78-89. doi: 10.17294/2330-0698.1418 Published quarterly by Midwest-based health system Advocate Aurora Health and indexed in PubMed Central, the Journal of Patient-Centered Research and Reviews (JPCRR) is an open access, peer-reviewed medical journal focused on disseminating scholarly works devoted to improving patient-centered care practices, health outcomes, and the patient experience. REVIEW Fungal Infections From Human and Animal Contact Dennis J. Baumgardner, MD Aurora University of Wisconsin Medical Group, Aurora Health Care, Milwaukee, WI; Department of Family Medicine and Community Health, University of Wisconsin School of Medicine and Public Health, Madison, WI; Center for Urban Population Health, Milwaukee, WI Abstract Fungal infections in humans resulting from human or animal contact are relatively uncommon, but they include a significant proportion of dermatophyte infections. Some of the most commonly encountered diseases of the integument are dermatomycoses. Human or animal contact may be the source of all types of tinea infections, occasional candidal infections, and some other types of superficial or deep fungal infections. This narrative review focuses on the epidemiology, clinical features, diagnosis and treatment of anthropophilic dermatophyte infections primarily found in North America. -
Hypertrichosis Terminalis
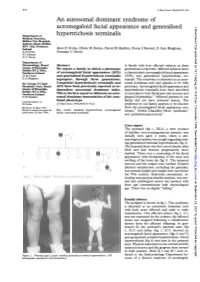
97297 Med Genet 1996;33:972-974 An autosomal dominant syndrome of acromegaloid facial appearance and generalised J Med Genet: first published as 10.1136/jmg.33.11.972 on 1 November 1996. Downloaded from Department of hypertrichosis terminalis Medical Genetics, Belfast City Hospital, Lisburn Road, Belfast BT9 7AB, Northern Ireland Alan D Irvine, Olivia M Dolan, David R Hadden, Fiona J Stewart, E Ann Bingham, A D Irvine Norman C Nevin F J Stewart N C Nevin Department of Dermatology, Royal Abstract A family with four affected subjects in three Group of Hospitals, Belfast BT12 6BA, We report a family in which a phenotype generations is reported. Affected subjects show Northern Ireland of acromegaloid facial appearance (AFA) a characteristic acromegaloid facial appearance 0 M Dolan and generalised hypertrichosis terminalis (AFA) and generalised hypertrichosis ter- E A Bingham segregates through three generations. minalis. The syndrome is inherited as an auto- Sir George E Clark Congenital hypertrichosis terminalis and somal dominant trait and appears to be fully Metabolic Unit, Royal AFA have been previously reported as in- penetrant. Acromegaloid facial appearance and Group of Hospitals, dependent autosomal dominant traits. hypertrichosis terminalis have been described Belfast BT12 6BA, Northern Ireland This is the first report to delineate an auto- in association with thickened oral mucosa and D R Hadden somal dominant transmission of the com- gingival hyperplasia.'"2 Affected patients in our Correspondence to: bined phenotype. family did not have intraoral lesions. The Dr Irvine. (JtMed Genet 1996;33:972-974) syndrome in our family appears to be discrete Received 14 May 1996 from the acromegaloid facial appearance syn- Revised version accepted for Key words: terminal hypertrichosis; acromegaloid drome,3 Gorlin-Chaudhry-Moss syndrome,4 publication 28 June 1996 facies; autosomal dominant. -

1 Structure and Function of the Skin
Go Back to the Top To Order, Visit the Purchasing Page for Details 1 Chapter 1 Structure and Function of the Skin The skin is the human body’s its largest organ, covering 1.6 m2 of surface area and accounting for approximate- ly 16% of an adult’s body weight. In direct contact with the outside environment, the skin helps to maintain four essential bodily functions: ① retention of moisture and prevention of permeation or loss of other molecules, ② regulation of body temperature, ③ protection of the body from microbes and harmful external influences, and ④ sensation. To understand cutaneous biology and skin diseases, it is very important to learn the structure and functions of normal human skin. A. Skin surface The skin surface is not smooth, but is laced with multiple net- works of fine grooves called sulci cutis. These can be deep or shallow. The slightly elevated areas that are surrounded by shal- lower areas of sulci cutis are called cristae cutis. Sweat pores fed crista cutis by the sweat glands open to the cristae cutis (Fig. 1.1). The orientation of the sulci cutis, which differs depending on body location, is called the dermal ridge pattern. Fingerprints and sulcus cutis patterns on the palms and soles, which are unique to each person, are formed by the sulci cutis. Elastic fibers also run in specific directions in deeper parts of the skin, with the direction depend- aabcdefg h i j klmnopqr ing on the site. Some skin diseases, such as epidermal nevus, are known to occur along specific lines distributed over the body, the Blaschko lines (Fig. -

Dermatology DDX Deck, 2Nd Edition 65
63. Herpes simplex (cold sores, fever blisters) PREMALIGNANT AND MALIGNANT NON- 64. Varicella (chicken pox) MELANOMA SKIN TUMORS Dermatology DDX Deck, 2nd Edition 65. Herpes zoster (shingles) 126. Basal cell carcinoma 66. Hand, foot, and mouth disease 127. Actinic keratosis TOPICAL THERAPY 128. Squamous cell carcinoma 1. Basic principles of treatment FUNGAL INFECTIONS 129. Bowen disease 2. Topical corticosteroids 67. Candidiasis (moniliasis) 130. Leukoplakia 68. Candidal balanitis 131. Cutaneous T-cell lymphoma ECZEMA 69. Candidiasis (diaper dermatitis) 132. Paget disease of the breast 3. Acute eczematous inflammation 70. Candidiasis of large skin folds (candidal 133. Extramammary Paget disease 4. Rhus dermatitis (poison ivy, poison oak, intertrigo) 134. Cutaneous metastasis poison sumac) 71. Tinea versicolor 5. Subacute eczematous inflammation 72. Tinea of the nails NEVI AND MALIGNANT MELANOMA 6. Chronic eczematous inflammation 73. Angular cheilitis 135. Nevi, melanocytic nevi, moles 7. Lichen simplex chronicus 74. Cutaneous fungal infections (tinea) 136. Atypical mole syndrome (dysplastic nevus 8. Hand eczema 75. Tinea of the foot syndrome) 9. Asteatotic eczema 76. Tinea of the groin 137. Malignant melanoma, lentigo maligna 10. Chapped, fissured feet 77. Tinea of the body 138. Melanoma mimics 11. Allergic contact dermatitis 78. Tinea of the hand 139. Congenital melanocytic nevi 12. Irritant contact dermatitis 79. Tinea incognito 13. Fingertip eczema 80. Tinea of the scalp VASCULAR TUMORS AND MALFORMATIONS 14. Keratolysis exfoliativa 81. Tinea of the beard 140. Hemangiomas of infancy 15. Nummular eczema 141. Vascular malformations 16. Pompholyx EXANTHEMS AND DRUG REACTIONS 142. Cherry angioma 17. Prurigo nodularis 82. Non-specific viral rash 143. Angiokeratoma 18. Stasis dermatitis 83. -

Nail Problems
Nail Problems Components of the Nail Congenital Disorders Racket nails, characterized by a broad short thumb nail, is the commonest congenital nail defect, dominantly inherited and seen in 1% of the population. The basic abnormality is shortness of the underlying terminal phalanx. In the yellow nail syndrome, the nail changes begin in adult life, against a background of hypoplasia of the lymphatic system. Peripheral edema is usually present and pleural effusions may occur. The nails grow very slowly and become thickened and greenish-yellow; their surface is smooth but they are over curved from side to side. Acquired Nail Changes Beau's Lines Transverse lines or grooves in nail. Causes include any severe systemic illness or medications (chemotherapy), which affects growth of the nail matrix. Clinically: The grooves or lines move distally; the distance from the nail fold lets one assess the time of trauma. Onycholysis Separation of nail from nail bed. Causes include psoriasis, dermatitis, fungal infections; medications (photo-onycholysis from tetracyclines or psoralens), thyroid disease; rarely inherited. Idiopathic onycholysis is most common among women; painless separation of nail without apparent cause. Typically, the distal third separates and underlying nail bed becomes darker and thickened. Therapy: Cut nail very short to reduce leverage encouraging separation, apply antifungal solution. Usually self-limited process. Ingrown Nail Penetration of nail plate into tissue of lateral nail fold. Almost always involves great toes. Causes include congenital malformation of nail (pincer nail), improper trimming, and tightly fitting shoes. Clinically: Distorted nail with swelling, pain, and granulation tissue along the lateral nail fold. Therapy: Mild cases: Eliminate pressure, trim nail; topical antiseptics as foot soaks or on small piece of cotton wool pushed under affected nail. -

Digital Clubbing
Review Article Digital clubbing Malay Sarkar, D. M. Mahesh1, Irappa Madabhavi2 Department of Pulmonary Medicine, Indira Gandhi Medical College, Shimla, 1Department of Endocrinology, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry, 2Post Graduate Student (Medicine), Indira Gandhi Medical College, Shimla, India ABSTRACT Digital clubbing is an ancient and important clinical signs in medicine. Although clubbed fingers are mostly asymptomatic, it often predicts the presence of some dreaded underlying diseases. Its exact pathogenesis is not known, but platelet‑derived growth factor and vascular endothelial growth factor are recently incriminated in its causation. The association of digital clubbing with various disease processes and its clinical implications are discussed in this review. KEY WORDS: Cancer, digital clubbing, hypertrophic osteoarthropathy, megakaryocytes, vascular endothelial growth factor Address for correspondence: Dr. Malay Sarkar, Department of Pulmonary Medicine, Indira Gandhi Medical College, Shimla ‑ 171 001, India. E‑mail: [email protected] INTRODUCTION long bones and occasional painful joint enlargement. It was initially known as hypertrophic pulmonary Digital clubbing is characterized by a focal bulbous osteoarthropathy (HPOA) based on the fact that majority enlargement of the terminal segments of the fingers of cases of HOA are due to malignant thoracic tumors. and/or toes due to proliferation of connective tissue The term “pulmonary” was later abandoned as it was between nail matrix and the distal phalanx. It results in realized that the skeletal syndrome may occur in several increase in both anteroposterior and lateral diameter of non‑pulmonary diseases and even may occur without the nails.[1] Clubbed fingers are also known as watch‑glass any underlying illness. -

Fundamental Shoulder Exercises
FUNDAMENTAL SHOULDER EXERCISES RANGE OF MOTION EXERCISES 1. L-BAR FLEXION Lie on back and grip L-Bar between index finger and thumb, elbows straight. Raise both arms overhead as far as possible keeping thumbs up. Hold for _____ seconds and repeat _____ times. 2. L-BAR EXTERNAL ROTATION, SCAPULAR PLANE Lie on back with involved arm 450 from body and elbow bent at 900. Grip L-Bar in the hand of involved arm and keep elbow in flexed position. Using unin- volved arm, push involved arm into external rotation. Hold for _____ seconds, return to starting position. Repeat _____ times. 3. L-BAR INTERNAL ROTATION, SCAPULAR PLANE Lie on back with involved arm 450 from body and elbow bent at 900. Grip L-Bar in the hand of involved arm and keep elbow in flexed position. Using the uninvolved arm, push involved arm into internal rotation. Hold for _____ seconds, return to starting position. Repeat _____ times. Dr. Meisterling (800) 423-1088 1 of 2 STRENGTHENING EXERCISES 1. TUBING, EXTERNAL ROTATION Standing with involved elbow fixed at side, elbow bent to 900 and involved arm across the front of the body. Grip tubing handle while the other end of tubing is fixed. Pull out with arm, keeping elbow at side. Return tubing slowly and controlled. Perform _____ sets of _____ reps. 2. TUBING, INTERNAL ROTATION Standing with elbow at side fixed at 900 and shoulder rotated out. Grip tubing handle while other end of tubing is fixed. Pull arm across body keeping elbow at side. Return tubing slowly and controlled. -

Rethinking the Evolution of the Human Foot: Insights from Experimental Research Nicholas B
© 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb174425. doi:10.1242/jeb.174425 REVIEW Rethinking the evolution of the human foot: insights from experimental research Nicholas B. Holowka* and Daniel E. Lieberman* ABSTRACT presumably owing to their lack of arches and mobile midfoot joints Adaptive explanations for modern human foot anatomy have long for enhanced prehensility in arboreal locomotion (see Glossary; fascinated evolutionary biologists because of the dramatic differences Fig. 1B) (DeSilva, 2010; Elftman and Manter, 1935a). Other studies between our feet and those of our closest living relatives, the great have documented how great apes use their long toes, opposable apes. Morphological features, including hallucal opposability, toe halluces and mobile ankles for grasping arboreal supports (DeSilva, length and the longitudinal arch, have traditionally been used to 2009; Holowka et al., 2017a; Morton, 1924). These observations dichotomize human and great ape feet as being adapted for bipedal underlie what has become a consensus model of human foot walking and arboreal locomotion, respectively. However, recent evolution: that selection for bipedal walking came at the expense of biomechanical models of human foot function and experimental arboreal locomotor capabilities, resulting in a dichotomy between investigations of great ape locomotion have undermined this simple human and great ape foot anatomy and function. According to this dichotomy. Here, we review this research, focusing on the way of thinking, anatomical features of the foot characteristic of biomechanics of foot strike, push-off and elastic energy storage in great apes are assumed to represent adaptations for arboreal the foot, and show that humans and great apes share some behavior, and those unique to humans are assumed to be related underappreciated, surprising similarities in foot function, such as to bipedal walking. -

Dermoscopy of Aplasia Cutis Congenita: a Case Report and Review of the Literature
Dermatology Practical & Conceptual Dermoscopy of Aplasia Cutis Congenita: A Case Report and Review of the Literature Rasna Neelam1, Mio Nakamura2, Trilokraj Tejasvi2 1 University of Michigan Medical School, Ann Arbor, MI, USA 2 Department of Dermatology, University of Michigan, Ann Arbor, MI, USA Key words: aplasia cutis congenita, alopecia, dermoscopy, trichoscopy Citation: Neelam R, Nakamura M, Tejasvi T. Dermoscopy of aplasia cutis congenita: a case report and review of the literature. Dermatol Pract Concept. 2021;11(1):e2021154. DOI: https://doi.org/10.5826/dpc.1101a154 Accepted: September 28, 2020; Published: January 29, 2021 Copyright: ©2021 Neelam et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License BY-NC-4.0, which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: None. Competing interests: The authors have no conflicts of interest to disclose. Authorship: All authors have contributed significantly to this publication. Corresponding author: Trilokraj Tejasvi, MD, Department of Dermatology, University of Michigan, 1910 Taubman Center, 1500 E. Medical Center Dr, Ann Arbor, MI 48109, USA Email: [email protected] Introduction throbbing, and point tenderness in one of the patches of alo- pecia. The alopecic patch had been present on the right pari- Aplasia cutis congenita (ACC) is a rare heterogeneous con- etal-occipital scalp since birth and has been stable in size and genital disorder characterized by focal or widespread absence appearance for years. Three other round patches of alopecia of the skin. had also been present since birth and remained asymptomatic. -

NAIL DISEASES and NAIL HEALTH Your Nails Can Tell You a Lot About Your Health
Dermatology Patient Education NAIL DISEASES AND NAIL HEALTH Your nails can tell you a lot about your health. Nail diseases and warning signs of other health problems appear on the nails. Your nails also reveal whether you are taking good care them. Good nail care is important because it can help prevent many common nail problems. NAIL DISEASES The skin around our nails and the tissue beneath are susceptible to many diseases. If you see any of the following, promptly see a dermatologist. Early diagnosis and proper treatment offer the best outcome. If allowed to progress, nail disease can be challenging to treat. Melanoma under the nail • Dark spot or streak Melanoma (skin cancer): Nail streaks are common in people of color. While many nail streaks are harmless, it is important to know that about 30% to 40% of melanomas that occur in people of color develop under a nail. While melanoma under the nail is more common in people of color, anyone can get melanoma under a nail. If your nail has a dark streak or spot and you do not remember injuring the nail, promptly see a dermatologist. When caught early, melanoma can be cured. • Growth Skin cancer: Many different types of skin cancer, including melanoma and Squamous cell carcinoma, can form under or around a nail. If you see a growth under or around your nail, promptly see a dermatologist. Your dermatologist can tell you whether the growth should be removed. Wart: A growth on the skin surrounding a nail is often a wart. Warts are common on the hands and feet.