Article
Evaluating Feruloyl Esterase—Xylanase Synergism for Hydroxycinnamic Acid and Xylo-Oligosaccharide Production from Untreated, Hydrothermally Pre-Treated and Dilute-Acid Pre-Treated Corn Cobs
- Lithalethu Mkabayi 1 , Samkelo Malgas 1 , Brendan S. Wilhelmi 2 and Brett I. Pletschke 1,
- *
1
Enzyme Science Programme (ESP), Department of Biochemistry and Microbiology, Rhodes University, Grahamstown, Eastern Cape 6140, South Africa; [email protected] (L.M.); [email protected] (S.M.) Department of Biochemistry and Microbiology, Rhodes University, Grahamstown, Eastern Cape 6140,
2
South Africa; [email protected]
*
Correspondence: [email protected]; Tel.: +27-46-6038081
Received: 4 April 2020; Accepted: 30 April 2020; Published: 13 May 2020
Abstract: Agricultural residues are considered the most promising option as a renewable feedstock
for biofuel and high valued-added chemical production due to their availability and low cost.
The efficient enzymatic hydrolysis of agricultural residues into value-added products such as sugars
and hydroxycinnamic acids is a challenge because of the recalcitrant properties of the native biomass.
Development of synergistic enzyme cocktails is required to overcome biomass residue recalcitrance,
and achieve high yields of potential value-added products. In this study, the synergistic action of two termite metagenome-derived feruloyl esterases (FAE5 and FAE6), and an endo-xylanase (Xyn11) from
Thermomyces lanuginosus, was optimized using 0.5% (w/v) insoluble wheat arabinoxylan (a model
substrate) and then applied to 1% (w/v) corn cobs for the efficient production of xylo-oligosaccharides
(XOS) and hydroxycinnamic acids. The enzyme combination of 66% Xyn11 and 33% FAE5 or FAE6
(protein loading) produced the highest amounts of XOS, ferulic acid, and p-coumaric acid from
untreated, hydrothermal, and acid pre-treated corn cobs. The combination of 66% Xyn11 and 33%
FAE6 displayed an improvement in reducing sugars of approximately 1.9-fold and 3.4-fold for
hydrothermal and acid pre-treated corn cobs (compared to Xyn11 alone), respectively. The hydrolysis
product profiles revealed that xylobiose was the dominant XOS produced from untreated and
pre-treated corn cobs. These results demonstrated that the efficient production of hydroxycinnamic
acids and XOS from agricultural residues for industrial applications can be achieved through the
synergistic action of FAE5 or FAE6 and Xyn11. Keywords: Ferulic acid; Feruloyl esterase; Xylanase; Synergy; Xylo-oligosaccharides
1. Introduction
Lignocellulosic biomass is widely considered one of the most promising low-cost feedstocks for the production of renewable energy and value-added chemicals. It is primarily composed of
three major components, namely: cellulose (40–60%), hemicellulose (20–40%), and lignin (10–25%) [1].
Waste agricultural residues generated during crop harvesting and processing (e.g., rice straw, wheat
straw, corn stover, corn cobs, sugarcane bagasse, sorghum bagasse, etc.) are renewable biomass
resources that are readily available and inexpensive [2]. A significant amount of research has focused
on developing environmentally friendly methods of utilising lignocellulosic biomass. In this regard,
enzymatic conversion has emerged as a major technological platform that offers several advantages
Agronomy 2020, 10, 688
2 of 14
such as environmental benefits and lower energy costs, with no formation of undesirable by-products.
Due to the complexity and heterogeneity in the structure of biomass, its enzymatic conversion requires
the synergistic cooperation of several enzymes [3]. A detailed understanding of this synergistic
cooperation is key in the development of enzyme cocktails for the optimal production of value-added
chemicals from lignocellulosic biomass.
Feruloyl esterases (FAEs, EC 3.1.1.73) and endo-xylanases (EC 3.2.1.8) are essential enzymes for
the degradation of xylan, the most abundant hemicellulose in biomass. FAEs catalyze the cleavage of covalent ester linkages between hydroxycinnamic acids and polysaccharides, releasing ferulic acid (FA) and p-coumaric acid (p-CA) from lignocellulosic biomass [ hydroxycinnamic acid, is usually esterified at the C-5 hydroxy group of the arabinofuranosyl units of arabinoxylan of commelinid plants [ ]. Hydroxycinnamic acids are used in the food industry as
precursors for vanillin, p-hydroxybenzoic acid and 4-vinylphenol production, and as food preservatives
because of their antimicrobial properties [ ], while in the pharmaceutical industry, they can be used
for their antioxidant and anti-inflammatory properties [ 11]. Application of FAEs not only releases
4,5]. FA, the most abundant
6
7
8–
hydroxycinnamic acid, but also decreases biomass recalcitrance, making it more accessible for further
hydrolysis by carbohydrate-active enzymes [11]. Endo-xylanases are essential in degrading xylan as
they catalyze the random cleavage of β-1,4-D-xylosidic linkages, generating xylo-oligosaccharides
(XOS) [12]. The enzymes have been classified into glycoside hydrolase (GH) families 5, 8, 10, 11, 30,
43, 62 and 98, with GH10 and 11 being the two families that have been extensively characterized (www.cazy.org). Endo-xylanase generated XOS from agricultural residues are used in food, feed,
and pharmaceutical industries due to their prebiotic and antioxidant activity [13,14].
FAEs exhibit a synergistic interaction with xylanases during the hydrolysis of a range of lignocellulosic substrates, which is demonstrated by improved yields in the production of XOS and FA. Xylanases generate ferulated XOS, which become preferred substrates for FAEs to cleave
ester bonds from, liberating FA as a product [15]. The removal of FA then increases the accessibility
of xylanases to XOS for further hydrolysis into shorter XOS and/or xylose. Studies have reported
significant increases in the amount of FA released from arabinoxylan after the enzymatic hydrolysis by
FAEs in the presence of xylanases [16–18]. Although FAEs from different microorganisms have been
used for the co-production of FA and XOS, some of these FAEs show limited hydrolysis efficiencies.
Therefore, novel FAEs with exceptional catalytic properties are still required for the formulation of
more efficient enzyme cocktails.
Rashamuse and co-workers [19] functionally screened novel FAEs from the hindgut prokaryotic
symbionts of Trinervitermes trinervoides termite species, but the application of these enzymes in
degrading agricultural residues has not yet been explored. In this study, the synergistic action of two
new termite metagenome derived FAEs (FAE5 and FAE6) and a GH11 xylanase from Thermomyces
lanuginosus was optimized on insoluble wheat arabinoxylan (model substrate) and then applied to corn
cobs (a natural substrate) for the production of XOS and hydroxycinnamic acids. The study presented
demonstrated that high quantities of XOS, p-CA and FA were generated from corn cobs as a result of synergistic interactions between Xyn11 and FAE5 or FAE6.
2. Materials and Methods
2.1. Chemicals, Substrates and Enzymes
Escherichia coli BL21 (DE3) was used for the expression of fae5 and fae6 genes harboured in pET28
plasmids. The cells were cultured in Luria-Bertani (LB) medium containing 50 µg/mL kanamycin at
37 ◦C. The induction was conducted by following the method described previously [20]. Enzymes were purified using 5 mL Ni-NTA Superflow Cartridges (QIAGEN®), purchased from QIAGEN (Germany),
as per the manufacturer’s instructions. Xylanase (Xyn11) from Thermomyces lanuginosus and ethyl ferulate (EFA) were purchased from Sigma Aldrich (South Africa). Insoluble wheat arabinoxylan
(WAX) was purchased from Megazyme™ (Ireland).
Agronomy 2020, 10, 688
3 of 14
2.2. Pre-Treatment of Corn Cobs
Milled corn cobs (CC) was treated using hydrothermal pre-treatment or dilute acid pre-treatment.
A total of 10 g of biomass was suspended in Milli-Q water (for hydrothermal treatment) or in a 0.5%
(w/w) sulphuric acid solution (for dilute acid treatment) (solid: liquid ratio of 1:10) and autoclaved for
20 min at 121 ◦C. The pre-treated CC slurry was filtered, followed by washing the solids repeatedly
with Milli-Q water and then oven drying to constant weight at 50 ◦C for 48 h.
2.3. Chemical Characterization of CC
The total carbohydrates of CC were determined in triplicate by using a modified sulphuric acid
method described previously [21]. Briefly, 300 mg of CC (untreated and pre-treated) was hydrolyzed
with 72% (v/v) sulphuric acid at 30 ◦C for 1 h, diluted to 3% (v/v) sulphuric acid and autoclaved to
solubilize the carbohydrate fraction. Following the hydrolysis, fractions were filtered to remove the
insoluble lignin from the solution.
Determination of the alkali-extractable hydroxycinnamic acid content was carried out by treating
10 mg of biomass with 1 M NaOH solution for 24 h at room temperature and in the dark. The liquors
obtained from alkaline treatments were separated from the solid fraction by centrifugation at 16,000×
g
for 5 min. The liquors were neutralized by 2 volumes of 1 M HCl and analyzed by HPLC as described
in Section 2.4.
The morphological structure of untreated and pre-treated biomass was analyzed with a scanning
electron microscope (SEM), JOEL JSM 840. CC samples were mounted on a metal stub with adhesive
tape and coated with a thin layer of gold prior to SEM analysis.
In order to detect changes in functional groups, FTIR analysis of untreated and pre-treated CC was
conducted by loading a few milligrams of pulverized samples in the universal ATR of a Spectrum 100
FT-IR spectrometer system (Perkin Elmer, Wellesley, MA). FT-IR spectra were recorded in quadruple at
- a range of 650–4000 cm−1 with a resolution of 4 cm−1
- .
2.4. Determination of Enzyme Activities and Protein Concentration
For feruloyl esterase activity assay, ethyl ferulate (EFA) was used as substrate. The reaction (1 mL) was carried out in sodium phosphate buffer (50 mM, pH 7.4) that contained 1 mM EFA. The reaction was initiated by the addition of a diluted enzyme solution. After 15 min incubation at 40 ◦C, the enzymatic
activity was terminated by incubation at 100 ◦C for 5 min. The released FA from the substrate was quantified using a Shimadzu HPLC system (Shimadzu Corp, Japan) equipped with a diode array
detector (DAD), where the chromatographic separation was achieved using a Phenomenex® C18 5
(150 4.6 mm) LC column (Phenomenex, United States of America). Ambient conditions were used
µm
×
for analysis. The mobile phase A was 0.01 M phosphoric acid and the mobile phase B consisted of
HPLC grade acetonitrile. The isocratic mobile phase consisted of A: 70% and B: 30% and ran for 10 min at a flow rate of 0.8 mL/min. The injected volume was 10 µL and the UV absorption of the effluent was
monitored at 320 nm. One unit of enzyme activity was defined as the amount of enzyme that released
1 µmol of ferulic acid per min under standard conditions.
For xylanase activity assay, 10 mg/mL of insoluble wheat arabinoxylan (WAX) was used as a substrate. The reaction mixture consisted of 300 µL of 1.33% (w/v) substrate dissolved in 50 mM sodium phosphate buffer (pH 7.4) and 100 µL of diluted enzyme. After 15 min incubation at 40 ◦C,
the enzymatic activity was terminated by incubation at 100 ◦C for 5 min, followed by centrifugation at
16,000
×
g for 5 min. The concentrations of reducing sugar were measured using 3,5-dinitrosalicylic
- L of the sample was mixed with 300 L of
- acid (DNS) method described previously [22]. Briefly, 150
- µ
- µ
DNS followed by boiling for 5 min. One unit of enzyme activity was defined as the amount of enzyme
that released 1 µmol of reducing sugars per min. Reducing sugars were estimated using a xylose
standard curve. The yield of reducing sugars was determined using the following equation:
- XOS yield (%) = ((XOS released × 0.88)/ xylan content) × 100
- (1)
Agronomy 2020, 10, 688
4 of 14
The Bradford method was used to determine the protein concentration of the enzymes [23].
A protein standard curve was constructed using bovine serum albumin as a suitable standard.
2.5. Synergy Studies
In order to study the synergistic interactions on agricultural residues, the FAE and Xyn11
combinations were first tested on WAX for the release of FA and XOS. Enzyme loadings for all single
enzymes and all enzyme combinations were kept at a total protein loading of 4 mg protein per g of
WAX and at 8 mg per g of CC. For combination experiments, an enzyme mixture consisting of Xyn11
and FAE in a 66:33% protein ratio was used. The enzymatic hydrolysis was performed at a substrate
loading of 0.5% (w/v) for WAX and 1% (w/v) for CC in 50 mM phosphate buffer (pH 7.4) in a total
volume of 400 µL. The reaction mixture was incubated at 40◦C with agitation at 25 rpm for 24 h and
terminated by boiling at 100 ◦C for 5 min. Hydrolysis controls included reactions without the addition of enzyme or substrate. All the experiments were performed in triplicate. The amount of FA and p-CA
released was determined using the HPLC method described in Section 2.4, while reducing sugars were
measured using the DNS method and XOS were quantified using the HPLC-RID method described in
Section 2.7.
2.6. Determination of Xylo-Oligosaccharides Pattern Profiles
In order to determine the hydrolysis product profiles from synergy studies, XOS were analyzed
by thin-layer chromatography (TLC). Five µL of hydrolysate sample and a mixture of XOS standards
were applied on a silica gel 60 F254 plate (Merck, Darmstadt, Germany). The migration was repeated
twice using a mobile phase consisting of 1-butanol, acetic acid and water in a 2:1:1 ratio, respectively.
The plate was then submerged in Molisch’s Reagent (0.3% (w/v) α-naphthol dissolved in methanol and
sulphuric acid in a 95:5 ratio (v/v), respectively). The spots corresponding to the different XOS were
visualized by heating the plate in the oven at 110 ◦C for 10 min.
The XOS were quantified by a Shimadzu HPLC system (Shimadzu Corp, Japan) equipped with
a refractive index detector (RID) using a CarboSep CHO 411 column (Anatech, South Africa) with water as the mobile phase in isocratic mode. The column oven was set at 80 ◦C and separation was
performed within 35 min at a flow rate of 0.3 mL per min. An injection volume of 20
for all samples and XOS standards.
µL was employed
2.7. Statistical Analysis
All statistical analyses were performed on GraphPad Prism 6.0 software using the t-test. A p-value
of less than 0.05 was considered to indicate statistically significant differences between compared
data sets.
3. Results
3.1. Chemical Characterization of CC
The main hurdle in utilising lignocellulosic biomass lies in its recalcitrant nature due to the
complexity of its biomass structure. Thus, to increase the enzymatic hydrolysis of biomass, various
pre-treatment strategies are usually required. However, some of the pre-treatment methods are known
for easily solubilizing some of the polysaccharides, mostly hemicellulose [24]. It is, therefore, critical
to perform pre-treatment under conditions that lead to a recovery of the biomass components in a re-usable form while increasing the enzymatic digestibility. In this study, hydrothermal and dilute
acid pre-treatment strategies were selected. An extremely low thermo-chemical pre-treatment severity
was applied in order to preserve hemicellulose and hydroxycinnamic content in the solid fraction.
In order to determine the effect and efficiency of the pre-treatments, the surface morphology of CC was
visualized with SEM. Figure 1 shows the SEM micrographs of untreated and pre-treated CC.
Agronomy 2020, 10, 688
5 of 14
- (a)
- (b)
- (c)
Figure 1. Morphological study of corn cobs (CC) by SEM. SEM micrographs of (a) untreated,
(b) hydrothermal treated, and (c) acid-treated at a 2k× magnification.
Here it can be seen that the untreated sample appears to possess a compact structure. In contrast,
the micrographs of pre-treated CC samples display distorted and fragmented structures on the surface.
The structural changes due to pre-treatment might increase the surface area of pre-treated CC, which
could lead to an enhanced enzymatic degradability.
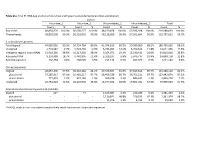
To determine the recoverable sugars and hydroxycinnamic acids after pre-treatment, the chemical composition of the untreated and pre-treated CC was also analyzed (Table 1). For noting,
the composition of other CC components such as lignin was omitted, and attention was focused on
glucan, xylan, arabinan, and hydroxycinnamic acids. The content of glucan, xylan, and arabinan
was slightly higher in the pre-treated biomass compared to untreated biomass samples. There were
more recoverable sugars in the hydrothermal treated sample compared to the acid pre-treated sample.
A slight increase in FA and p-CA content was observed in the acid-treated sample. The data on Table 1
confirmed that the relevant CC components were successfully retained upon pre-treatment.
Table 1. Chemical composition of untreated and pre-treated CC (on a percentage dry mass basis).
Reducing
- Glucan a
- Xylan a
- Arabinan a
- FA c
- p-CA c
Sugars b
Untreated
Hydrothermal treated
Acid-treated
30.86 ± 0.90 36.03 ± 0.13 32.58 ± 0.85
11.46 ± 0.48 13.47 ± 0.91 12.22 ± 0.48
7.79 ± 0.63 11.24 ± 0.84 8.52 ± 0.55
53.00 ± 0.49 62.38 ± 0.29 59.20 ± 0.15
0.61± 0.012 0.68 ± 0.027 0.68 ± 0.027
0.63 ± 0.026 0.61 ± 0.024 0.67 ± 0.013
a
Analysis method: Megazyme sugar kits, b DNS method, c HPLC. The data presented are averages
±
standard
deviations of triplicates.
To further investigate the chemical changes that took place during the pre-treatment of CC,
FTIR analysis was conducted. The spectra of untreated, hydrothermal and acid-treated CC are shown in Figure 2. The absorption peaks at around 1730 cm−1 region are predominantly attributed to the C=O stretching vibration of the ester linkage of the carboxylic group of FA and p-CA of
lignin and/or hemicellulose [25]. The spectra of all samples show this peak suggesting that changes
due to pre-treatment (observed in SEM micrographs) did not lead to the removal of hemicellulose and hydroxycinnamic acids. The FTIR data is in agreement with composition analysis (Table 1),
as the hydrothermal pre-treated sample displayed strong absorption bands around 1000 cm−1 and in