2016 Table of Drugs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Depo-Estradiol (Estradiol Cypionate) Injection
Market Applicability Market DC GA KY MD NJ NY WA Applicable X X X X X X NA Depo-Estradiol (estradiol cypionate) injection Override(s) Approval Duration Prior Authorization 1 year Medications Depo-Estradiol (estradiol cypionate) injection APPROVAL CRITERIA Requests for Depo-Estradiol (estradiol cypionate) injection may be approved when the following criteria are met: I. Individual is using to treat moderate to severe vasomotor symptoms associated with menopause; OR II. Individual has been diagnosed with hypoestrogenism due to hypogonadism; OR III. Individual has a diagnosis of gender dysphoria or gender identity disorder (WPATH 2012, Endocrine Society 2017) ; AND IV. Individual is 16 years of age or older; AND V. The goal of treatment is male-to-female gender reassignment. Requests for Depo-Estradiol (estradiol cypionate) injection may not be approved for any of the following: I. Individual has undiagnosed abnormal genital bleeding; OR II. Individual has known or suspected cancer of the breast; OR III. Individual has known or suspected estrogen-dependent neoplasia; OR IV. Individual has active deep vein thrombosis, pulmonary embolism or history of these conditions; OR V. Individual has active or recent (within the past year) arterial thromboembolic disease (such as stroke or myocardial infarction); OR VI. Individual has liver dysfunction or disease. PAGE 1 of 2 02/24/2020 New Program Date 02/24/2020 CRX-ALL-0519-20 This policy does not apply to health plans or member categories that do not have pharmacy benefits, nor does it apply to Medicare. Note that market specific restrictions or transition-of-care benefit limitations may apply. -

Pms-ESTRADIOL VALERATE INJECTION
PRODUCT MONOGRAPH Prpms-ESTRADIOL VALERATE INJECTION Estradiol Valerate 10 mg/mL Estrogen PHARMASCIENCE INC. Date of Revision: 6111 Royalmount Avenue, Suite 100 June 30, 2009 Montreal, Quebec H4P 2T4 Control Number: 120632 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION……………………………...3 SUMMARY PRODUCT INFORMATION……………………………………….3 INDICATIONS AND CLINICAL USE…………………………………………...3 CONTRAINDICATIONS…………………………………………………………4 WARNINGS AND PRECAUTIONS…………………………………….……….5 ADVERSE REACTIONS………………………………………………………...13 DRUG INTERACTIONS………………………………………………………...15 DOSAGE AND ADMINISTRATION…………………………………………...17 OVERDOSAGE………………………………………………………………….19 ACTION AND CLINICAL PHARMACOLOGY……………………………….19 STORAGE AND STABILITY…………………………………………………...22 DOSAGE FORMS, COMPOSITION AND PACKAGING……………………..22 PART II: SCIENTIFIC INFORMATION…………………………………………….23 PHARMACEUTICAL INFORMATION………………………………………...23 CLINICAL TRIALS……………………………………………………………...24 DETAILED PHARMACOLOGY………………………………………………..24 REFERENCES…………………………………………………………………...25 PART III: CONSUMER INFORMATION……………………………………………………27 2 PRODUCT MONOGRAPH Prpms- ESTRADIOL VALERATE INJECTION (Estradiol Valerate) 10 mg/mL PART I: HEALTH PROFESSIONAL INFORMATION SUMMARY PRODUCT INFORMATION Route of Dosage Form / Strength Clinically Relevant Nonmedicinal Administration Ingredients Intramuscular Injection / 10 mg/mL Sesame Oil For a complete listing see Dosage Forms, Composition and Packaging section. INDICATIONS AND CLINICAL USE pms-ESTRADIOL VALERATE INJECTION is indicated in the treatment of: I. amenorrhea (primary -

Estradiol-17Β Pharmacokinetics and Histological Assessment Of
animals Article Estradiol-17β Pharmacokinetics and Histological Assessment of the Ovaries and Uterine Horns following Intramuscular Administration of Estradiol Cypionate in Feral Cats Timothy H. Hyndman 1,* , Kelly L. Algar 1, Andrew P. Woodward 2, Flaminia Coiacetto 1 , Jordan O. Hampton 1,2 , Donald Nickels 3, Neil Hamilton 4, Anne Barnes 1 and David Algar 4 1 School of Veterinary Medicine, Murdoch University, Murdoch 6150, Australia; [email protected] (K.L.A.); [email protected] (F.C.); [email protected] (J.O.H.); [email protected] (A.B.) 2 Faculty of Veterinary and Agricultural Sciences, University of Melbourne, Melbourne 3030, Australia; [email protected] 3 Lancelin Veterinary Hospital, Lancelin 6044, Australia; [email protected] 4 Department of Biodiversity, Conservation and Attractions, Locked Bag 104, Bentley Delivery Centre 6983, Australia; [email protected] (N.H.); [email protected] (D.A.) * Correspondence: [email protected] Received: 7 September 2020; Accepted: 17 September 2020; Published: 21 September 2020 Simple Summary: Feral cats (Felis catus) have a devastating impact on Australian native fauna. Several programs exist to control their numbers through lethal removal, using tools such as baiting with toxins. Adult male cats are especially difficult to control. We hypothesized that one way to capture these male cats is to lure them using female cats. As female cats are seasonal breeders, a method is needed to artificially induce reproductive (estrous) behavior so that they could be used for this purpose year-round (i.e., regardless of season). -

Drug and Chemical Reference Guideline
WISCONSIN DEPARTMENT OF AGRICULTURE, TRADE AND CONSUMER PROTECTION DIVISION OF FOOD AND RECREATIONAL SAFETY TITLE: Drug and Chemical Reference Guideline Document #: Replaces: New Revision Date: 1/21/2020 Page 1 of 6 General Guidelines: These drugs and substances are not to be used or • Drugs and administration equipment stored to not stored on dairy farms. These drugs are not eligible for contaminate milk or milk contact surfaces ELU privileges by veterinarians. • Non-lactating drugs separated from lactating drugs • Chloramphenicol using separate shelves or cabinets • Clenbuterol • Drugs properly labeled (see label requirements • Colloidal Silver below) • Diethylstilbestrol (DES) • Locked drug cabinets must be made accessible for • Dimethysulfoxide (DMSO) inspection • Dimetridazole • Drugs for animal species other than cattle must not • Dipyrone (Novine, No Pain) be stored in dairy facility • Estradiol Cypionate (ECP) • All bottles or packages in case lots properly labeled • Fluoroquinolones (sarafloxacin-Saraflox, orbafloxacin-Orbox, danofloxacin mesylate-A 180) Drug Labeling Requirements: • Furazolidone, Nitrofurazone, other Nitrofurans OTC Drugs: (over the counter) • Glycopeptides (vancomycin) • Name of Drug • Iononphores (Lasalocid) permitted for non-lactating • Active ingredients • Ipronidazole • Directions for use • Metronidazole and other Nitroimidazoles • Withholding/withdrawal or discard time for meat or • Phenylbutazone, “bute” (prohibited in dairy animals milk (even if zero) 20 months of age or older - no ELU allowed.) • Name -

Drug Code List
HCPCS/Drug Code List Version 13.2 Revised 6/1/21 List will be updated routinely Disclaimer: For drug codes that require an NDC, coverage depends on the drug NDC status (rebate eligible, Non-DESI, non-termed, etc) on the date of service. Note: Physician/Facility-administered medications are reimbursed using the Centers for Medicare and Medicaid Services (CMS) Part B Drug pricing file found on the CMS website--www.cms.gov. In the absence of a fee, pricing may reflect the methodolgy used for retail pharmacies. Go to data.medicaid.gov for a complete list of drug NDCs participating in the Medicaid drug rebate program. Consult with each Managed Care Organization (MCO) about their coverage guidelines and prior authroization requirements, if applicable. Highlights represent updated material for each specific revision of the Drug Code List. Code Description Brand Name NDC NDC unit Category Service AC CAH P NP MW MH HS PO OPH HI ID DC Special Instructions req. of Limits OP OP TF for measure drug rebate ? 90281 human ig, im Gamastan Yes ML Antisera NONE X X X X Closed 3/31/13. 90283 human ig, iv Gamimune, Yes ML Antisera NONE X X X X Closed 3/31/13. Cost invoice required with claim. Restricted to ICD-9 diagnoses codes 204.10 - 204.12, Flebogamma, 279.02, 279.04, 279.06, 279.12, 287.31, and 446.1, and must be included on claim form, effective 10/1/09. Gammagard 90287 botulinum antitoxin N/A Antisera Not Covered 90288 botulism ig, iv No ML NONE X X X X Requires documentation and medical review 90291 cmv ig, iv Cytogam Yes ML Antisera NONE X X X X Closed 3/31/13. -

Pharmacological Management of Acromegaly: a Current Perspective
Neurosurg Focus 29 (4):E14, 2010 Pharmacological management of acromegaly: a current perspective SUNIL MANJILA , M.D.,1 Osmo N D C. WU, B.A.,1 FAH D R. KHAN , M.D., M.S.E.,1 ME H ree N M. KHAN , M.D.,2 BAHA M. AR A F AH , M.D.,2 AN D WA rre N R. SE L M AN , M.D.1 1Department of Neurological Surgery, The Neurological Institute, and 2Division of Clinical and Molecular Endocrinology, University Hospitals Case Medical Center, Cleveland, Ohio Acromegaly is a chronic disorder of enhanced growth hormone (GH) secretion and elevated insulin-like growth factor–I (IGF-I) levels, the most frequent cause of which is a pituitary adenoma. Persistently elevated GH and IGF-I levels lead to substantial morbidity and mortality. Treatment goals include complete removal of the tumor causing the disease, symptomatic relief, reduction of multisystem complications, and control of local mass effect. While trans- sphenoidal tumor resection is considered first-line treatment of patients in whom a surgical cure can be expected, pharmacological therapy is playing an increased role in the armamentarium against acromegaly in patients unsuitable for or refusing surgery, after failure of surgical treatment (inadequate resection, cavernous sinus invasion, or transcap- sular intraarachnoid invasion), or in select cases as primary treatment. Three broad drug classes are available for the treatment of acromegaly: somatostatin analogs, dopamine agonists, and GH receptor antagonists. Somatostatin analogs are considered as the first-line pharmacological treatment of acromegaly, although effica- cy varies among the different formulations. Octreotide long-acting release (LAR) appears to be more efficacious than lanreotide sustained release (SR). -

To Download a List of Prescription Drugs Requiring Prior Authorization
Essential Health Benefits Standard Specialty PA and QL List July 2016 The following products require prior authorization. In addition, there may be quantity limits for these drugs, which is notated below. Therapeutic Category Drug Name Quantity Limit Anti-infectives Antiretrovirals, HIV SELZENTRY (maraviroc) None Cardiology Antilipemic JUXTAPID (lomitapide) 1 tab/day PRALUENT (alirocumab) 2 syringes/28 days REPATHA (evolocumab) 3 syringes/28 days Pulmonary Arterial Hypertension ADCIRCA (tadalafil) 2 tabs/day ADEMPAS (riociguat) 3 tabs/day FLOLAN (epoprostenol) None LETAIRIS (ambrisentan) 1 tab/day OPSUMIT (macitentan) 1 tab/day ORENITRAM (treprostinil diolamine) None REMODULIN (treprostinil) None REVATIO (sildenafil) Soln None REVATIO (sildenafil) Tabs 3 tabs/day TRACLEER (bosentan) 2 tabs/day TYVASO (treprostinil) 1 ampule/day UPTRAVI (selexipag) 2 tabs/day UPTRAVI (selexipag) Pack 2 packs/year VELETRI (epoprostenol) None VENTAVIS (iloprost) 9 ampules/day Central Nervous System Anticonvulsants SABRIL (vigabatrin) pack None Depressant XYREM (sodium oxybate) 3 bottles (540 mL)/30 days Neurotoxins BOTOX (onabotulinumtoxinA) None DYSPORT (abobotulinumtoxinA) None MYOBLOC (rimabotulinumtoxinB) None XEOMIN (incobotulinumtoxinA) None Parkinson's APOKYN (apomorphine) 20 cartridges/30 days Sleep Disorder HETLIOZ (tasimelteon) 1 cap/day Dermatology Alkylating Agents VALCHLOR (mechlorethamine) Gel None Electrolyte & Renal Agents Diuretics KEVEYIS (dichlorphenamide) 4 tabs/day Endocrinology & Metabolism Gonadotropins ELIGARD (leuprolide) 22.5 mg -

Somatostatin Analogues in the Treatment of Neuroendocrine Tumors: Past, Present and Future
International Journal of Molecular Sciences Review Somatostatin Analogues in the Treatment of Neuroendocrine Tumors: Past, Present and Future Anna Kathrin Stueven 1, Antonin Kayser 1, Christoph Wetz 2, Holger Amthauer 2, Alexander Wree 1, Frank Tacke 1, Bertram Wiedenmann 1, Christoph Roderburg 1,* and Henning Jann 1 1 Charité, Campus Virchow Klinikum and Charité, Campus Mitte, Department of Hepatology and Gastroenterology, Universitätsmedizin Berlin, 10117 Berlin, Germany; [email protected] (A.K.S.); [email protected] (A.K.); [email protected] (A.W.); [email protected] (F.T.); [email protected] (B.W.); [email protected] (H.J.) 2 Charité, Campus Virchow Klinikum and Charité, Campus Mitte, Department of Nuclear Medicine, Universitätsmedizin Berlin, 10117 Berlin, Germany; [email protected] (C.W.); [email protected] (H.A.) * Correspondence: [email protected]; Tel.: +49-30-450-553022 Received: 3 May 2019; Accepted: 19 June 2019; Published: 22 June 2019 Abstract: In recent decades, the incidence of neuroendocrine tumors (NETs) has steadily increased. Due to the slow-growing nature of these tumors and the lack of early symptoms, most cases are diagnosed at advanced stages, when curative treatment options are no longer available. Prognosis and survival of patients with NETs are determined by the location of the primary lesion, biochemical functional status, differentiation, initial staging, and response to treatment. Somatostatin analogue (SSA) therapy has been a mainstay of antisecretory therapy in functioning neuroendocrine tumors, which cause various clinical symptoms depending on hormonal hypersecretion. Beyond symptomatic management, recent research demonstrates that SSAs exert antiproliferative effects and inhibit tumor growth via the somatostatin receptor 2 (SSTR2). -

PHOTOSTENT-02: Porfimer Sodium Photodynamic Therapy Plus Stenting Versus Stenting Alone in Patients with Locally Advanced Or Metastatic Biliary Tract Cancer
Open access Original research ESMO Open: first published as 10.1136/esmoopen-2018-000379 on 23 July 2018. Downloaded from PHOTOSTENT-02: porfimer sodium photodynamic therapy plus stenting versus stenting alone in patients with locally advanced or metastatic biliary tract cancer Stephen P Pereira,1,2 Mark Jitlal,3 Marian Duggan,3 Emma Lawrie,3 Sandy Beare,3 Pam O'Donoghue,4 Harpreet S Wasan,5 Juan W Valle,6 John Bridgewater,7 on behalf of the PHOTOSTENT-02 investigators To cite: Pereira SP, ABSTRACT Key questions Jitlal M, Duggan M, et al. Background Endobiliary stenting is standard practice for PHOTOSTENT-02: porfimer palliation of obstructive jaundice due to biliary tract cancer sodium photodynamic therapy What is already known about this subject? (BTC). Photodynamic therapy (PDT) may also improve plus stenting versus stenting In patients with obstructive jaundice due to unre- biliary drainage and previous small studies suggested ► alone in patients with locally sectable cholangiocarcinoma, small studies have survival benefit. advanced or metastatic biliary suggested that photodynamic therapy (PDT) may Aims To assess the difference in outcome between tract cancer. ESMO Open improve biliary drainage and patient survival. 2018;3:e000379. doi:10.1136/ patients with BTC undergoing palliative stenting plus PDT esmoopen-2018-000379 versus stenting alone. What does this study add? Methods 92 patients with confirmed locally advanced ► We conducted a large randomised controlled trial or metastatic BTC, ECOG performance status 0–3 and of porfimer sodium PDT in patients with confirmed JWV and JB contributed equally. adequate biliary drainage were randomised (46 per locally advanced or metastatic biliary tract cancer. -

Regulatory Mechanisms of Somatostatin Expression
International Journal of Molecular Sciences Review Regulatory Mechanisms of Somatostatin Expression Emmanuel Ampofo * , Lisa Nalbach, Michael D. Menger and Matthias W. Laschke Institute for Clinical & Experimental Surgery, Saarland University, 66421 Homburg/Saar, Germany; [email protected] (L.N.); [email protected] (M.D.M.); [email protected] (M.W.L.) * Correspondence: [email protected]; Tel.: +49-6841-162-6561; Fax: +49-6841-162-6553 Received: 25 May 2020; Accepted: 9 June 2020; Published: 11 June 2020 Abstract: Somatostatin is a peptide hormone, which most commonly is produced by endocrine cells and the central nervous system. In mammals, somatostatin originates from pre-prosomatostatin and is processed to a shorter form, i.e., somatostatin-14, and a longer form, i.e., somatostatin-28. The two peptides repress growth hormone secretion and are involved in the regulation of glucagon and insulin synthesis in the pancreas. In recent years, the processing and secretion of somatostatin have been studied intensively. However, little attention has been paid to the regulatory mechanisms that control its expression. This review provides an up-to-date overview of these mechanisms. In particular, it focuses on the role of enhancers and silencers within the promoter region as well as on the binding of modulatory transcription factors to these elements. Moreover, it addresses extracellular factors, which trigger key signaling pathways, leading to an enhanced somatostatin expression in health and disease. Keywords: somatostatin; pre-prosomatostatin; δ-cells; central nervous system (CNS); gut; hypothalamus; cAMP resonse element (CRE); pancreas/duodenum homeobox protein (PDX)1; paired box protein (PAX)6; growth hormone (GH); brain-derived neurotrophic factor (BDNF); glutamateric system; pancreas 1. -
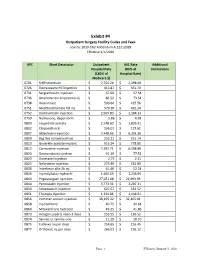
Exhibit #4 Outpatient Surgery Facility Codes and Fees Source: 2019 CN2 Addendum A.12212018 Effective 1/1/2020
Exhibit #4 Outpatient Surgery Facility Codes and Fees source: 2019 CN2 Addendum A.12212018 Effective 1/1/2020 APC Short Descriptor Outpatient ASC Rate Additional Hospital Rate (85% of Instructions (180% of Hospital Rate) Medicare $) 0701 Sr89 strontium $ 2,704.24 $ 2,298.60 0726 Dexrazoxane HCl injection $ 413.87 $ 351.79 0731 Sargramostim injection $ 67.69 $ 57.54 0736 Amphotericin b liposome inj $ 86.52 $ 73.54 0738 Rasburicase $ 500.66 $ 425.56 0751 Mechlorethamine hcl inj $ 579.10 $ 492.24 0752 Dactinomycin injection $ 2,569.80 $ 2,184.33 0759 Naltrexone, depot form $ 5.86 $ 4.98 0800 Leuprolide acetate $ 2,148.60 $ 1,826.31 0802 Etoposide oral $ 136.01 $ 115.61 0807 Aldesleukin injection $ 7,448.66 $ 6,331.36 0809 Bcg live intravesical vac $ 253.11 $ 215.14 0810 Goserelin acetate implant $ 916.24 $ 778.80 0812 Carmustine injection $ 7,292.71 $ 6,198.80 0820 Daunorubicin injection $ 91.19 $ 77.51 0823 Docetaxel injection $ 2.72 $ 2.31 0825 Nelarabine injection $ 273.99 $ 232.89 0836 Interferon alfa-2b inj $ 61.46 $ 52.24 0840 Inj melphalan hydrochl $ 1,466.65 $ 1,246.65 0843 Pegaspargase injection $ 27,051.68 $ 22,993.93 0844 Pentostatin injection $ 3,773.31 $ 3,207.31 0850 Streptozocin injection $ 627.67 $ 533.52 0851 Thiotepa injection $ 1,334.84 $ 1,134.61 0856 Porfimer sodium injection $ 38,195.22 $ 32,465.94 0858 Inj cladribine $ 40.75 $ 34.64 0864 Mitoxantrone hydrochl $ 49.25 $ 41.86 0873 Hyalgan supartz visco-3 dose $ 153.55 $ 130.52 0874 Synvisc or synvisc-one $ 21.29 $ 18.10 0875 Euflexxa inj per dose $ 254.65 $ 216.45 0877 -

Oregon Health Authority Division of Medical Assistance Programs Addendum a - Final OPPS Apcs for CY 2012 Effective October 1, 2012
Oregon Health Authority Division of Medical Assistance Programs Addendum A - Final OPPS APCs for CY 2012 Effective October 1, 2012 Relative APC Group Title SI Weight 0001 Level I Photochemotherapy S 0.5042 0002 Fine Needle Biopsy/Aspiration T 1.6115 0003 Bone Marrow Biopsy/Aspiration T 3.5702 0004 Level I Needle Biopsy/ Aspiration Except Bone Marrow T 4.5746 0005 Level II Needle Biopsy/Aspiration Except Bone Marrow T 8.1566 0006 Level I Incision & Drainage T 1.4206 0007 Level II Incision & Drainage T 13.1250 0008 Level III Incision and Drainage T 20.5648 0012 Level I Debridement & Destruction T 0.3878 0013 Level II Debridement & Destruction T 0.8785 0015 Level III Debridement & Destruction T 1.4989 0016 Level IV Debridement & Destruction T 2.7592 0017 Level V Debridement & Destruction T 21.6661 0019 Level I Excision/ Biopsy T 4.4238 0020 Level II Excision/ Biopsy T 8.2746 0021 Level III Excision/ Biopsy T 17.0074 0022 Level IV Excision/ Biopsy T 23.2662 0028 Level I Breast Surgery T 25.5054 0029 Level II Breast Surgery T 33.4070 0030 Level III Breast Surgery T 44.8999 0031 Smoking Cessation Services X 0.2997 0034 Mental Health Services Composite S 2.7295 0035 Vascular Puncture and Minor Diagnostic Procedures X 0.2691 0037 Level IV Needle Biopsy/Aspiration Except Bone Marrow T 15.3499 0039 Level I Implantation of Neurostimulator Generator S 216.7598 0040 Level I Implantation/Revision/Replacement of Neurostimulator Electrodes S 63.7616 0041 Level I Arthroscopy T 29.6568 0042 Level II Arthroscopy T 57.0137 0045 Bone/Joint Manipulation Under