September 24, 2020 Maggie Zheng, Regulatory Affairs Manager
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WEIHAI CITY COMMERCIAL BANK CO., LTD.* 威海市商業銀行股份有限公司* (A Joint Stock Company Incorporated in the People’S Republic of China with Limited Liability) (Stock Code: 9677)
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. WEIHAI CITY COMMERCIAL BANK CO., LTD.* 威海市商業銀行股份有限公司* (A joint stock company incorporated in the People’s Republic of China with limited liability) (Stock Code: 9677) ANNOUNCEMENT OF ANNUAL RESULTS FOR THE YEAR ENDED 31 DECEMBER 2020 The board of directors (the “Board”) of Weihai City Commercial Bank Co., Ltd.* (the “Bank”) hereby announces the audited annual results of the Bank and its subsidiary (the “Group”) for the year ended 31 December 2020. This announcement, containing the full text of the 2020 annual report of the Bank, complies with the relevant requirements of the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited in relation to information to accompany preliminary announcement of annual results. The Group’s final results for the year ended 31 December 2020 have been reviewed by the audit committee of the Bank. This results announcement will be published on the website of The Stock Exchange of Hong Kong Limited (www.hkexnews.hk) and the Bank’s website (www.whccb.com). The Bank’s 2020 annual report will be despatched to the holders of H shares of the Bank and published on the websites of The Stock Exchange of Hong Kong Limited and the Bank in due course. -

中国输美木制工艺品注册登记企业名单registered Producing
中国输美木制工艺品注册登记企业名单 Registered Producing Industries of Wooden Handicrafts of China Exported to the U.S. 注册登记编号 序号 所在省份 所在城市 企业名称 企业地址 Registered Number Province City Company Name Company Address Number 天津市北辰区双口镇双河村30号 天津津长工艺品加工厂 天津 天津 NO.30, SHUANGHE VILLAGE, 1 TIANJIN JINCHANG ARTS & 1206ZMC0087 TIANJIN TIANJIN SHUANGKOU TOWN, BEICHEN CRAFTS FACTORY DISTRICT, TIANJIN CITY, CHINA 天津市静海县利邦工艺制品厂 天津市静海区西翟庄镇西翟庄 天津 天津 2 TIANJIN JINGHAI LEBANG ARTS XIZHAI ZHUANG, JINGHAI COUNTRY 1204ZMC0004 TIANJIN TIANJIN CRAFTS CO.,LTD TIANJIN 天津市宁河县板桥镇田庄坨村外东侧 天津鹏久苇草制品有限公司 天津 天津 THE EAST SIDE OF TIANZHUANGTUO 3 TIANJIN PENGJIU REED PRODUCTS 1200ZMC0012 TIANJIN TIANJIN VILLAGE, BANQIAO TOWN, NINGHE CO.,LTD. COUNTY, TIANJIN, CHINA 河北百年巧匠文化传播股份有限公司 石家庄桥西区新石北路399号 河北 石家庄 4 HEBEI BAINIANQIAOJIANG NO.399, XINSHI NORTH ROAD, QIAOXI 1300ZMC9003 HEBEI SHIJIAZHUANG CULTURE COMMUNICATION INC. DISTRICT, SHIJIAZHUANG CITY 行唐县森旺工贸有限公司 河北省石家庄行唐县口头镇 河北 石家庄 5 XINGTANGXIAN SENWANG KOUTOU TOWN, XINGTANG COUNTY, 1300ZMC0003 HEBEI SHIJIAZHUANG INDUSTRY AND TRADING CO.,LTD SHIJIAZHUANG, HEBEI CHINA 唐山市燕南制锹有限公司 河北省滦南县城东 河北 唐山 6 TANGSHAN YANNAN EAST OF LUANNAN COUNTY, HEBEI, 1302ZMC0002 HEBEI TANGSHAN SHOVEL-MAKING CO.,LTD CHINA 唐山天坤金属工具制造有限公司 河北省唐山市滦南县唐乐公路南侧 河北 唐山 7 TANGSHAN TIANKUN METAL SOUTH OF TANGLE ROAD, LUANNAN 1302ZMC0008 HEBEI TANGSHAN TOOLS MAKING CO.,LTD COUNTY, HEBEI, CHINA 唐山市长智农工具设计制造有限公司 TANGSHAN CHANGZHI 河北省滦南县城东杜土村南 河北 唐山 8 AGRICULTURAL TOOLS SOUTH OF DUTU TOWN, LUANNAN 1302ZMC0031 HEBEI TANGSHAN DESIGNING AND COUNTY, HEBEI, CHINA MANUFACTURING CO.,LTD. 唐山腾飞五金工具制造有限公司 河北省滦南县宋道口镇 河北 唐山 9 TANGSHAN TENGFEI HARDWARE SONG DAO KOU TOWN, LUANNAN 1302ZMC0009 HEBEI TANGSHAN TOOLS MANUFACTURE CO.,LTD. COUNTY, HEBEI CHINA 河北省滦南县宋道口镇杜土村南 唐山舒适五金工具制造有限公司 河北 唐山 SOUTH OF DUTU, SONG DAO KOU 10 TANGSHAN SHUSHI HARDWARE 1302ZMC0040 HEBEI TANGSHAN TOWN, LUANNAN COUNTY, HEBEI TOOLS MANUFACTURE CO,LTD CHINA 唐山腾骥锻轧农具制造有限公司 河北省滦南县宋道口镇 河北 唐山 TANGSHAN TENGJI FORGED 11 SONG DAO KOU TOWN, LUANNAN 1302ZMC0011 HEBEI TANGSHAN AGRICULTURE IMPLEMENTS COUNTY, HEBEI CHINA MANUFACTURING CO.,LTD. -

Qilu Expressway Company Limited 齊魯高速公路股份有限公司
The Stock Exchange of Hong Kong Limited and the Securities and Futures Commission take no responsibility for the contents of this Application Proof, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this Application Proof. Application Proof of Qilu Expressway Company Limited 齊 魯 高 速 公 路 股 份 有 限 公 司 (the ‘‘Company’’) (a joint stock company incorporated in the People’s Republic of China with limited liability) WARNING The publication of this Application Proof is required by The Stock Exchange of Hong Kong Limited (the ‘‘Exchange’’) and the Securities and Futures Commission (the ‘‘SFC’’) solely for the purpose of providing information to the public in Hong Kong. This Application Proof is in draft form. The information contained in it is incomplete and is subject to change which can be material. By viewing this document, you acknowledge, accept and agree with Qilu Expressway Company Limited (the ‘‘Company’’), its sole sponsor, advisers or members of the underwriting syndicate that: (a) this document is only for the purpose of providing information about the Company to the public in Hong Kong and not for any other purposes. No investment decision should be based on the information contained in this document; (b) the publication of this document or supplemental, revised or replacement pages on the Exchange’s website does not give rise to any obligation of the Company, its sole sponsor, advisers or members of the underwriting syndicate to proceed with an offering in Hong Kong or any other jurisdiction. -

2019年第3季度在韩国注册的中国水产企业名单the List of Chinese
2019年第3季度在韩国注册的中国水产企业名单 The List of Chinese Registered Fishery Processing Establishments Export to Korea (Total 1347 , the third quarter of 2019,updated on 25 June, 2019) No. Est.No. 企业名称(中文) Est.Name 企业地址(中文) Est.Address 产品(Products) 北京市朝阳区崔各庄乡 The 23rd floor Sanyuan Property Jingmi Road 北京中洋环球金枪鱼有 1 1100/02010 Beijing Zhongyang Global Tuna Co.,Ltd 东辛店村京密路三元物 Dongxindian Village Cuigezhuang TownChaoyang Fishery Products 限公司 业院内23号楼 District Beijng 五洋海产(天津)有限 天津市塘沽区东江路 2 1200/02004 Ocean Products (Tian.Jin) Co., Ltd Dongjiang Road No.3849 Tanggu Tianjin Fishery Products 公司 3849号 欧盛实业(天津)有限 天津经济技术开发区渤 No.5, Bohai Road, Tianjin Economic & Technological 3 1200/02019 Ocean (Tianjin) Corporation Ltd. Fishery Products 公司 海路5号 Development Area, Tianjin 天津市颖明海湾食品有 天津市滨海新区中心渔 No.221 Yuehai RD., Binhai New Area Of The City 4 1200/02028 Tianjin Smart Gulf Foodstuffs Co.,Ltd. Fishery Products 限公司 港经济区悦海路221号 Center Fishing Port Economic Zone, Tianjin, China 天津市塘沽区海华水产 Tianjin Tanggu District Haihua Fishery Products Food 天津市塘沽区北塘镇水 No. 9, Shuichan Road, Beitang Town, Tanggu District, 5 1200/02048 Fishery Products 食品有限公司 Co., Ltd. 产路9号 Tianjin 天津百迅达进出口贸易 天津市津南区双桥河镇 South Dongnigu Village, Shuangqiaohe Town, Jinnan 6 1200/02063 Tianjin baixunda import and export trade Co., Ltd Fishery Products 有限公司 东泥沽村南 District, Tianjin, China 昌黎县筑鑫实业有限公 秦皇岛市昌黎县新开口 Economic Development Zone Xinkaikou Changli 7 1300/02228 Changli Zhuxin Enterprises Co., Ltd. Fishery Products 司 经济开发区 County Qinhuangdao 抚宁县渤远水产品有限 秦皇岛市抚宁县台营镇 Yegezhuang village taiying town funing county 8 1300/02229 Funing county boyuan aquatic products co.,ltd Fishery Products 公司 埜各庄村 Qinhuangdao city Hebei province 秦皇岛市江鑫水产冷冻 河北省秦皇岛北戴河新 Nandaihe Second District,Beidaihe New 9 1300/02236 Qinhuangdao Jiangxin Aquatic Food Products Co., Ltd. -

Supply Chain Strategic Alliances Partner Selection for Rizhao Port
International Journal of Scientific and Research Publications, Volume 4, Issue 11, November 2014 1 ISSN 2250-3153 Supply Chain Strategic Alliances Partner Selection for Rizhao Port Libin Guo School of Management, Qufu Normal University,Rizhao276826,China Abstract- Under the background of port supply chain strategic alliances,we clarify the current situation of Rizhao Port by SWOT analysis and find out the ST strategy for it, furthermore, we screen out the optimal cooperation partner for Rizhao Port under different alliance forms range from horizontal integration and vertical integration to the blended dynamic logistics alliance. Index Terms- Port Supply Chain; the SWOT Analysis; RiZhao Port; Strategical alliance I. INTRODUCTION ort supply chain, a main constituent of port logistics, includes the levels such as informationization, automation and networking, P and emphasizes the modern management of material transportation chain of each link and the extension of comprehensive services.Meanwhile, due to the connection between ports and suppliers and consumers all over the world through shippping companies and land forwarding agents, a port supply chain integrating many means1 of transportation and types of logistics is formed, and it becomes a relatively best main part and link for the coordinated management of supply chain. Therefore, we can start from the analysis of the SWOT of Rizhao port supply chain, and find out the cooperative partners suitable for the strategical alliance of Rizhao port supply chain among different types of alliance. II. THE SWOT ANALYSIS OF RIZHAO PORT SUPPLY CHAIN The SWOT analysis, a commonly used method of strategic competition, is based on analyzing the internal conditions of enterprises itself, and finds out the strengths, weaknesses and core competence. -

Table of Codes for Each Court of Each Level
Table of Codes for Each Court of Each Level Corresponding Type Chinese Court Region Court Name Administrative Name Code Code Area Supreme People’s Court 最高人民法院 最高法 Higher People's Court of 北京市高级人民 Beijing 京 110000 1 Beijing Municipality 法院 Municipality No. 1 Intermediate People's 北京市第一中级 京 01 2 Court of Beijing Municipality 人民法院 Shijingshan Shijingshan District People’s 北京市石景山区 京 0107 110107 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Haidian District of Haidian District People’s 北京市海淀区人 京 0108 110108 Beijing 1 Court of Beijing Municipality 民法院 Municipality Mentougou Mentougou District People’s 北京市门头沟区 京 0109 110109 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Changping Changping District People’s 北京市昌平区人 京 0114 110114 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Yanqing County People’s 延庆县人民法院 京 0229 110229 Yanqing County 1 Court No. 2 Intermediate People's 北京市第二中级 京 02 2 Court of Beijing Municipality 人民法院 Dongcheng Dongcheng District People’s 北京市东城区人 京 0101 110101 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Xicheng District Xicheng District People’s 北京市西城区人 京 0102 110102 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Fengtai District of Fengtai District People’s 北京市丰台区人 京 0106 110106 Beijing 1 Court of Beijing Municipality 民法院 Municipality 1 Fangshan District Fangshan District People’s 北京市房山区人 京 0111 110111 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Daxing District of Daxing District People’s 北京市大兴区人 京 0115 -
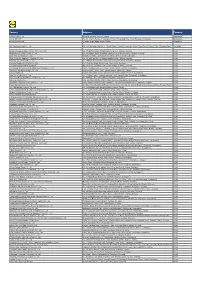
Factory Address Country
Factory Address Country Durable Plastic Ltd. Mulgaon, Kaligonj, Gazipur, Dhaka Bangladesh Lhotse (BD) Ltd. Plot No. 60&61, Sector -3, Karnaphuli Export Processing Zone, North Potenga, Chittagong Bangladesh Bengal Plastics Ltd. Yearpur, Zirabo Bazar, Savar, Dhaka Bangladesh ASF Sporting Goods Co., Ltd. Km 38.5, National Road No. 3, Thlork Village, Chonrok Commune, Korng Pisey District, Konrrg Pisey, Kampong Speu Cambodia Ningbo Zhongyuan Alljoy Fishing Tackle Co., Ltd. No. 416 Binhai Road, Hangzhou Bay New Zone, Ningbo, Zhejiang China Ningbo Energy Power Tools Co., Ltd. No. 50 Dongbei Road, Dongqiao Industrial Zone, Haishu District, Ningbo, Zhejiang China Junhe Pumps Holding Co., Ltd. Wanzhong Villiage, Jishigang Town, Haishu District, Ningbo, Zhejiang China Skybest Electric Appliance (Suzhou) Co., Ltd. No. 18 Hua Hong Street, Suzhou Industrial Park, Suzhou, Jiangsu China Zhejiang Safun Industrial Co., Ltd. No. 7 Mingyuannan Road, Economic Development Zone, Yongkang, Zhejiang China Zhejiang Dingxin Arts&Crafts Co., Ltd. No. 21 Linxian Road, Baishuiyang Town, Linhai, Zhejiang China Zhejiang Natural Outdoor Goods Inc. Xiacao Village, Pingqiao Town, Tiantai County, Taizhou, Zhejiang China Guangdong Xinbao Electrical Appliances Holdings Co., Ltd. South Zhenghe Road, Leliu Town, Shunde District, Foshan, Guangdong China Yangzhou Juli Sports Articles Co., Ltd. Fudong Village, Xiaoji Town, Jiangdu District, Yangzhou, Jiangsu China Eyarn Lighting Ltd. Yaying Gang, Shixi Village, Shishan Town, Nanhai District, Foshan, Guangdong China Lipan Gift & Lighting Co., Ltd. No. 2 Guliao Road 3, Science Industrial Zone, Tangxia Town, Dongguan, Guangdong China Zhan Jiang Kang Nian Rubber Product Co., Ltd. No. 85 Middle Shen Chuan Road, Zhanjiang, Guangdong China Ansen Electronics Co. Ning Tau Administrative District, Qiao Tau Zhen, Dongguan, Guangdong China Changshu Tongrun Auto Accessory Co., Ltd. -

Talent Sport Products Catalogue
Rizhao Talent Sport Products Co.,Ltd. Email: [email protected] Web: www.talentsportpro.com Room 1026, No.2 building of Antai International Square, Yantai Road, Qinlou Street, Donggang District, Rizhao City, Shandong Province, China CATALOGUE Version: 2020 Series Type Photo Material Size/Weight Color International Standard Usage 20" GP 4kg/9lbs - White - 30mm 6kg/13lbs - Grey - 30mm 8kg/18lbs - Pink - 32mm 10kg/22lbs - Orange - 32mm 12kg/26lbs - Blue - 32mm 14kg/31lbs - Brown - 32mm 16kg/35lbs - Yellow - 33mm 2kg,4kg,6kg,8kg,10kg,12kg, 18kg/40lbs - Brown - 33mm 14kg,16kg,18kg,20kg,22kg, Any color can be Fitness equipment, home, Cast Iron Kettlebell High Quality Cast Iron 20kg/44lbs - Grey - 38mm 22ton 24kg,26kg,28kg,32kg-64kg customed as request office, gym, yoga etc 22kg/48lbs - Black - 38mm by 2kg increase 24kg/53lbs - Green - 39mm 28kg/62lbs - Orange - 40mm 32kg/70lbs - Red - 40mm 36kg/80lbs - White - 40mm 40kg/88lbs - Blue - 40mm 44kg/97lbs - Brown - 40mm 48kg/106lbs - Black - 40mm 4KG-black, 6kg-light pink, 8kg-pink, 10kg-sky blue, 12kg-blue, 14kg-light brow, Kettlebell 4kg,6kg,8kg,10kg,12kg,14kg, 16kg -yellow, 18kg-light purple, 16kg,18kg,20kg,22kg,24kg, Any color can be Fitness equipment, home, Steel Competition Kettlebell Low Carbon Steel 20kg-purple, 22kg-light green, 22ton 26kg,28kg,32kg,36kg, 40kg, customed as request office, gym, yoga etc 24-green, 26kg-black, 44kg,48kg 28kg-orange, 32kg-red, 36kg-grey,40kg-white, 44kg-siver, 48kg-gold 12kg,14kg,16kg,18kg,20kg, Any color can be Fitness equipment, home, Adjustable Kettlebell High quality cast iron __ 22ton 22kg,24kg,26kg,28kg,32kg customed as request office, gym, yoga etc Rizhao Talent Sport Products Co.,Ltd. -

The World Bank
Document of The World Bank Public Disclosure Authorized Report No: ICR00003941 IMPLEMENTATION COMPLETION AND RESULTS REPORT (IBRD-78820) Public Disclosure Authorized ON A LOAN IN THE AMOUNT OF US$ 60 MILLION TO THE PEOPLE’S REPUBLIC OF CHINA Public Disclosure Authorized FOR A SHANDONG ECOLOGICAL AFFORESTATION PROJECT Jan. 31, 2017 Public Disclosure Authorized Environment and Natural Resources Global Practice China Country Office East Asia and Pacific Region CURRENCY EQUIVALENTS (Exchange Rate Effective August 2016) Currency Unit = Renminbi (RMB) Yuan RMB 1.00 = US$ 0.15 US$ 1.00 = RMB 6.47 FISCAL YEAR January 1 – December 31 ABBREVIATIONS AND ACRONYMS ACS Administrative and Client Support BP Bank Procedures CFB Country Forest Bureaus CPMO County Project Management Office CPS Country Partnership Strategy CO2 carbon dioxide DO development objective EA environmental assessment EACCF World Bank China Country Office EIB European Investment Bank EIRR Economic Internal Return Rate EMP Environmental Management Plan FIRR Financial Internal Return Rate GoC Government of China HS Highly Satisfactory Ha Hectares IA Implementation Agreement IBRD International Bank for Reconstruction and Development ICR Implementation Complementation and Results Report ISR Implementation Status and Results Report IP Implementation of Project M&E Monitoring & Evaluation MPMO Municipal Project Management Office MTR Mid-term Review OCC Opportunity Cost of Capital OP Operational Policy PAD Project Appraisal Document PES Payment for Environmental Services PCN Project Concept -

Emission Reduction Decision and Coordination of a Make-To-Order Supply Chain with Two Products Under Cap-And-Trade Regulation
Accepted Manuscript Emission reduction decision and coordination of a make-to-order supply chain with two products under cap-and-trade regulation Qingguo Bai, Jianteng Xu, Yingyu Zhang PII: S0360-8352(18)30123-2 DOI: https://doi.org/10.1016/j.cie.2018.03.032 Reference: CAIE 5136 To appear in: Computers & Industrial Engineering Received Date: 21 September 2017 Revised Date: 24 January 2018 Accepted Date: 19 March 2018 Please cite this article as: Bai, Q., Xu, J., Zhang, Y., Emission reduction decision and coordination of a make-to- order supply chain with two products under cap-and-trade regulation, Computers & Industrial Engineering (2018), doi: https://doi.org/10.1016/j.cie.2018.03.032 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Title Page Title: Emission reduction decision and coordination of a make-to-order supply chain with two products under cap-and-trade regulation Authors: Qingguo Bai, Jianteng Xu, Yingyu Zhang Author affiliations: School of Management, Qufu Normal University, Rizhao, 276826, China; Corresponding Author: Name: Qingguo Bai Address: School of Management, Qufu Normal University, No.80, Yantai Road, Donggang District, Rizhao City, Shandong Province, 276826, P.R.China E-mail: [email protected] [email protected] Telephone number: (86)13563337287 Acknowledgements: This work was supported by the National Science Foundation of China under Grant 71771138, Ministry of Education Foundation of Humanities and Social Sciences under Grant 17YJC630004, and National Science Foundation of Shandong Province, China under Grant ZR2017MG009. -

1 Latest Project on 13 July, the Group Acquired Zhong Cun Project in Qingdao. the Plot Is Located in Northeast Chengyang
HKSE Stock Code: 960 Latest Project On 13 July, the Group acquired Zhong Cun project in Qingdao. The plot is located in northeast Chengyang District, which is 30 km from the core downtown area of Qingdao and only 5 km from the Government of Chengyang District. The plot, with a total consideration of RMB475 million, has a site area of 210,500 sq.m., offers a GFA of 302,700 sq.m. and has an accommodation value of RMB1,569 per sq.m.. The plot will be developed into an integrated community incorporating townhouses, villas, high-rises apartment and shops. On 26 July, the Group acquired Dong Gang project in Dalian. The plot is located in the center of the CBD of Donggang District, Dalian. Close Chongqing Chunsen Starry Street to a metro station and it is the only residential project in the region that has a magnificent sea view. The plot, with a total consideration of RMB1,652 million, has a site area of 62,800 sq.m., offers a GFA of 188,400 sq.m. and has an accommodation value of RMB8,767 per sq.m.. The plot will be developed into a high-end integrated community incorporating low-rises apartments, high-rises apartment and commercial centers. On 8 August, the Group acquired Li Jia project in Chongqing at reserve Chongqing Times Paradise Walk price. The plot is in the core area of the Lijia business district in the north area of Chongqing New Distric, with a total consideration of RMB4,220 million, has a site area of 879,000 sq.m, offers a GFA of 1,985,000 sq.m. -

Store Name:Microsoft Authorized Store Store ID: AR106 Contact
Store Name:Microsoft Authorized Store Store ID: AR106 Contact Name: Deng Hongbo Phone: 18953380824 Address: Floor 5, ZiBo Shopping Store, No.125 JinJing Street, ZhangDian District, Zibo, Shandong Province Postal Code: 255000 Store Name:Microsoft Authorized Store -Shandong Yinhao Information Technology Co., Ltd Store ID: AR146 Contact Name: Zheng Chenxiao Phone: 13370514476 Address: Q2007 Microsoft Store, No.1 gate of Huaqiang electronic world, Shanda Road, Lixia District, Jinan City Postal Code: 250013 Store ID: DSD01 Contact Name: Liu Jianqi Phone: 0534-2444111 Address: North Campus of No.5 Middle School, Lujia Street, Decheng District, Dezhou City, 100 meters to the east of the road Postal Code: 253000 Store ID: DSD02 Contact Name: Zhang Yu Phone: 0546-8555163 Address: No. 28, Xisan Road, Dongying District, Dongying City (2nd floor, 20 meters north of Industrial and Commercial Bank of North China, intersection of Xisan Road and Jinan Road) Postal Code: 257061 Store ID: DSD05 Contact Name: Xia Yang Phone: 0531-86421116 Address: Rm. 1115, Keyuan Building, Shanda Road Postal Code: 250014 Store ID: DSD06 Contact Name: Long Maoqiang Phone: 0537-2905079 Address: Jinyu road and road intersection southwest corner of pipa Postal Code: 272100 Store ID: DSD07 Contact Name: Yan Jiabao Phone: 0539-8367282 Address: West of Cross road of Tongda Road Postal Code: 276000 Store ID: DSD08 Contact Name: Geng Haifeng Phone: 0532-80687682 Address: Rm. 1507, Yinjie Shouzuo,153 Liaoning Road Postal Code: 266300 Store ID: DSD09 Contact Name: Liu Wei Phone: 0538-8251091 Address: West Xiaheqiao, Caiyuan Ave. Postal Code: 271000 Store ID: DSD10 Contact Name: Liu Chengkai Phone: 0536-2996911 Address: North Temple Street Broadcasting Digital Plaza, six floor 619 Postal Code: 261041 Store ID: DSD11 Contact Name: Lu Haiying Phone: 0631-5285566 Address: No.