Recovery from Tachyphylaxis of TRPV1 Coincides with Recycling to the Surface Membrane
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MCQ Base Clinical Pharmacology
MCQ Base Clinical Pharmacology Mechanism of drug action is explored by: A) pharmacokinetics B) pharmacogenetics C) pharmacoeconomics D) pharmacodynamics E) pharmacognosy Therapeutic window is the dosages of a medication (therapeutic serum concentrations ) between: A) TD50 curve and ED50 B) ED50 and T1/2 C) the amount that gives an effect (effective dose) and the amount that gives more adverse effects than desired effects D) the amount that gives minimal adverse effects and the amount that gives more adverse effects E) the amount that gives minimal effect and the amount that gives full therapeutic effect Therapeutic index is the ratio of: A) LD50 over the ED50 B) ED50over the LD50 C) bioavailability over drug dose D) apparent volume of distribution over elimination rate constant E) total clearance over nonrenal (extrarenal) clearance Therapeutic drug monitoring means: A) trough concentration under steady-state condition B) measurement of medication concentrations in blood C) the process of chemical alteration of drugs in the body D) amount of untoward effects following treatment E) development of expected desired effects Therapeutic dose is not related to: A) patient’s age B) rout of administration C) desired therapeutic effect D) organs of elimination E) treatment costs Mean therapeutic doses mentioned in manuals is obtained by the following way: A) calculation of pharmacokinetic features B) clinical investigations C) experimental investigations D) experimental data adopted for human body E) calculation of pharmacodynamic features Find -

Evidence-Based Guidelines for Treating Depressive Disorders with Antidepressants
JOP0010.1177/0269881115581093Journal of PsychopharmacologyCleare et al. 581093research-article2015 BAP Guidelines Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 2008 British Association Journal of Psychopharmacology 2015, Vol. 29(5) 459 –525 for Psychopharmacology guidelines © The Author(s) 2015 Reprints and permissions: sagepub.co.uk/journalsPermissions.nav DOI: 10.1177/0269881115581093 jop.sagepub.com Anthony Cleare1, CM Pariante2 and AH Young3 With expert co-authors (in alphabetical order): IM Anderson4, D Christmas5, PJ Cowen6, C Dickens7, IN Ferrier8, J Geddes9, S Gilbody10, PM Haddad11, C Katona12, G Lewis12, A Malizia13, RH McAllister-Williams14, P Ramchandani15, J Scott16, D Taylor17, R Uher18 and the members of the Consensus Meeting19 Endorsed by the British Association for Psychopharmacology Abstract A revision of the 2008 British Association for Psychopharmacology evidence-based guidelines for treating depressive disorders with antidepressants was undertaken in order to incorporate new evidence and to update the recommendations where appropriate. A consensus meeting involving experts in depressive disorders and their management was held in September 2012. Key areas in treating depression were reviewed and the strength of evidence and clinical implications were considered. The guidelines were then revised after extensive feedback from participants and interested parties. A literature review is provided which identifies the quality of evidence upon which the recommendations -

First Part Examination Mock Exam
FIRST PART EXAMINATION MOCK EXAM This Mock Examination is prepared to provide candidates, tutors and their Supervisors of Training with information about the way the Examiners will assess the performance of candidates in the Examination. Example questions provided are to be used as a guide only as to what may be expected. Candidates should discuss the exam with their tutors so that they may prepare appropriately for future examinations. It should be read in conjunction with the Notes to Candidates document. WRITTEN EXAM GLOSSARY OF TERMS Calculate Work out or estimate using mathematical principles Classify Divide into categories; organise, arrange Compare Examine similarities and differences Define Give the precise meaning Describe Give a detailed account of Explain Make plain, interpret, and account for Interpret Explain the meaning or significance Outline Provide a summary of the important points Relate Show a connection between Understand Appreciate the details of; comprehend 1 FIRST PART EXAMINATION MOCK EXAM SHORT ANSWER QUESTIONS MORNING PAPER (A) Start each answer on a new page and indicate the question number. It is not necessary to rewrite the question in your answer book. (B) You should aim to allocate ten minutes to answer each SAQ. (C) The questions are worth equal marks. (D) Short Answer Questions with more than one part have the proportion of marks indicated for each part. (E) Record your candidate number and each question number on the cover of each book and hand in all answer books. 2 Please answer Questions 1 and 2 in the blue answer booklet 1. Describe the anatomy of the left subclavian vein. -

(DMT), Harmine, Harmaline and Tetrahydroharmine: Clinical and Forensic Impact
pharmaceuticals Review Toxicokinetics and Toxicodynamics of Ayahuasca Alkaloids N,N-Dimethyltryptamine (DMT), Harmine, Harmaline and Tetrahydroharmine: Clinical and Forensic Impact Andreia Machado Brito-da-Costa 1 , Diana Dias-da-Silva 1,2,* , Nelson G. M. Gomes 1,3 , Ricardo Jorge Dinis-Oliveira 1,2,4,* and Áurea Madureira-Carvalho 1,3 1 Department of Sciences, IINFACTS-Institute of Research and Advanced Training in Health Sciences and Technologies, University Institute of Health Sciences (IUCS), CESPU, CRL, 4585-116 Gandra, Portugal; [email protected] (A.M.B.-d.-C.); ngomes@ff.up.pt (N.G.M.G.); [email protected] (Á.M.-C.) 2 UCIBIO-REQUIMTE, Laboratory of Toxicology, Department of Biological Sciences, Faculty of Pharmacy, University of Porto, 4050-313 Porto, Portugal 3 LAQV-REQUIMTE, Laboratory of Pharmacognosy, Department of Chemistry, Faculty of Pharmacy, University of Porto, 4050-313 Porto, Portugal 4 Department of Public Health and Forensic Sciences, and Medical Education, Faculty of Medicine, University of Porto, 4200-319 Porto, Portugal * Correspondence: [email protected] (D.D.-d.-S.); [email protected] (R.J.D.-O.); Tel.: +351-224-157-216 (R.J.D.-O.) Received: 21 September 2020; Accepted: 20 October 2020; Published: 23 October 2020 Abstract: Ayahuasca is a hallucinogenic botanical beverage originally used by indigenous Amazonian tribes in religious ceremonies and therapeutic practices. While ethnobotanical surveys still indicate its spiritual and medicinal uses, consumption of ayahuasca has been progressively related with a recreational purpose, particularly in Western societies. The ayahuasca aqueous concoction is typically prepared from the leaves of the N,N-dimethyltryptamine (DMT)-containing Psychotria viridis, and the stem and bark of Banisteriopsis caapi, the plant source of harmala alkaloids. -

Safety Alert: Risks Associated with Ophthalmic Anesthetics
WRHA Pharmacy Program Health Sciences Centre MS-189 820 Sherbrook St. Winnipeg, Manitoba R3A 1R9 CANADA TEL: 204-787-7183 Fax: 204-787-3195 FAX: 204-787-3195 Safety Alert: Risks associated with Ophthalmic Anesthetics The self-administration of ophthalmic anesthetics by patients for the relief of eye pain should be avoided and they should not be given to patients to take home for pain relief. Vision threatening complications of topical anesthetic abuse are common. There is no indication for the use of ophthalmic anesthetics except for diagnostic and short term therapeutic purposes (the removal of a foreign body or ocular surgery) and therefore, these products should only be used under a physician’s supervision. Eye trauma resulting in a corneal abrasion (epithelial injury) is a common complaint in the Emergency department (1). A superficial corneal injury can cause intense pain causing a patient to seek medical help or immediate relief from available over the counter remedies. In Canada, only two topical ophthalmic anesthetic drugs are available commercially as single entities, proparacaine (proxymetacaine) and tetracaine (available in bottle and minim forms). Benoxinate (oxybuprocaine) is only available in combination with fluorescein (3). Lidocaine is also used in ophthalmic surgical procedures however, it is not available in the Canadian market as an ophthalmic preparation. Topical ophthalmic anesthetics function by blocking nerve conduction when applied to the cornea and conjunctiva. The ocular surface is innervated by the multiple branches of the trigeminal nerve. The cornea is supplied by the long and short ciliary nerves, the nasociliary nerve and the lacrimal nerve (4). Topical anesthetics reduce sodium permeability preventing generation and conduction of nerve impulses, increasing excitation threshold, and slowing the nerve impulse propagation. -

Receptor Antagonist
Gut: first published as 10.1136/gut.29.7.890 on 1 July 1988. Downloaded from Gut, 1988, 29, 890-893 Alteration of H2 receptor sensitivity in duodenal ulcer patients after maintenance treatment with an H2 receptor antagonist D B JONES, C W HOWDEN, D W BURGET, CINDY SILLETTI, AND R H HUNT From the Division of Gastroenterology, McMaster University Medical Centre, Hamilton, Ontario, Canada SUMMARY The effects of a specific H2 receptor agonist impromidine, on gastric acid secretion were measured in six patients with duodenal ulcer in clinical remission before and after three months treatment with ranitidine 150 mg nocte. After treatment basal acid output increased from 1 2 to 2-8 mmol/h and after maximal impromidine stimulation from 36-9 (4 7) to 44-2 (6 2) mmol/h (p<002). Intravenous ranitidine 50 mg was given at the end ofthe impromidine infusion on each study day; the antisecretory effect of intravenous ranitidine was accentuated after the treatment with ranitidine from a trough acid output of8 5 (1-2) mmol/h before, to 3-8 (1-5) mmol/h (p<0 05) after, treatment. The increased response to the H2 agonist impromidine and the H2 antagonist ranitidine after treatment with ranitidine suggests an enhanced sensitivity of the H2 receptor. This might be ex- plained on the basis of an increase in the number of H2 receptors ('up-regulation'). http://gut.bmj.com/ The discovery of the histamine H2 receptor on the a highly potent, and specific agonist for the H2 parietal cell' initiated the development of H2 receptor' exhibiting up to 27 times the affinity of receptor antagonists with potent gastric antisecretory histamine.9 In this study we have examined the effect activity and these agents have now become firmly of three months of treatment with ranitidine 150 mg established in the treatment of peptic ulceration.- nocte on impromidine stimulated gastric acid secre- After heaing of duodenal ulcer by the H2 receptor tion in six patients with duodenal ulcer in remission. -

Npp2013281.Pdf
Neuropsychopharmacology (2013) 38, S435–S593 & 2013 American College of Neuropsychopharmacology. All rights reserved 0893-133X/13 www.neuropsychopharmacology.org Poster Session III-Wednesday participants indicated their ongoing experience of the Wednesday, December 11, 2013 intensity of heartbeat and breathing sensations by rotating a dial. Discrimination was measured by a positive dial deflection above baseline during an infusion trial. Accuracy W2. Interoceptive Awareness in Meditators During was measured via maximum cross correlation between each Cardiorespiratory Deviations in Body Arousal subject’s dial ratings and their corresponding heart rate Sahib Khalsa*, David Rudrauf, Richard Davidson, response. After each infusion participants also traced the Daniel Tranel body locations where they had felt heartbeat sensations, on a manikin template. UCLA Semel Institute for Neuroscience and Human Results: Bolus isoproterenol infusions elicited equivalent Behavior, Los Angeles, California increases in heart rate in both groups (Repeated measures ANOVA; F(1, 5) ¼ 50.1, po0.0001). There were no signifi- Background: Attention directed towards internal body cant group (F(1, 28) ¼ 1.27, p ¼ 0.27) or group x dose sensations is commonly practiced in many meditation interactions (F(1, 5) ¼ 0.04, p ¼ 0.998). There were also no traditions, and is a core skill cultivated when learning group differences in adrenergic sensitivity as measured by mindfulness. This practice is commonly proposed to CD25 (dose required to elevate heart rate by 25 beats per -

International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on Terms and Symbols in Quantitative Pharmacology
0031-6997/03/5504-597–606$7.00 PHARMACOLOGICAL REVIEWS Vol. 55, No. 4 Copyright © 2003 by The American Society for Pharmacology and Experimental Therapeutics 30404/1114803 Pharmacol Rev 55:597–606, 2003 Printed in U.S.A International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on Terms and Symbols in Quantitative Pharmacology RICHARD R. NEUBIG, MICHAEL SPEDDING, TERRY KENAKIN, AND ARTHUR CHRISTOPOULOS Department of Pharmacology, University of Michigan, Ann Arbor, Michigan (R.R.N.); Institute de Recherches Internationales Servier, Neuilly sur Seine, France (M.S.); Systems Research, GlaxoSmithKline Research and Development, Research Triangle Park, North Carolina (T.K.); and Department of Pharmacology, University of Melbourne, Parkville, Australia (A.C.) Abstract ............................................................................... 597 I. Introduction............................................................................ 597 II. Working definition of a receptor .......................................................... 598 III. Use of drugs in definition of receptors or of signaling pathways ............................. 598 A. The expression of amount of drug: concentration and dose ............................... 598 Downloaded from 1. Concentration..................................................................... 598 2. Dose. ............................................................................ 598 B. General terms used to describe drug action ........................................... -

ABX-2 Newslet: Cystitis & Skin
Antibiotics & Common Infections ABX-2: Uncomplicated Cystitis & Skin Stewardship, Effectiveness, Safety & Clinical Pearls- April 2017 ABX-2 RELATED LINKS RxFILES ACADEMIC DETAILING ON ABX CANADIAN GUIDELINES/REFERENCES We are excited to bring out the ABX-2 topic on the treatment of uncomplicated cystitis Bugs & Drugs: and skin & soft tissue (SSTI) infections. The new charts in this newsletter will support http://www.bugsanddrugs.ca/ our spring academic detailing discussions with providers in Saskatchewan. Our MUMS Guidelines: discussions on ABX-1 were very well received and we know many of you made use of the http://www.mumshealth.com extra support tools such as the “Gone Viral?” office/clinic posters and the patient friendly “Viral Prescription Pad”. These are all available at www.RxFiles.ca/abx. CYSTITIS / UTI U.S. IDSA 2010: ABX-2: A FEW PEARLS FROM INSIDE THAT CAUGHT OUR EYE... Acute Uncomplicated Cystitis and Pylonephritis (UTI) UNCOMPLICATED CYSTISIS - Page 2 - 3 > 60 YEARS https://academic.oup.com/cid/article- lookup/52/5/e103 1) Staying Power: > 60 years & still 96% or better! Susceptibility of E. coli, the most common urinary pathogen, to nitrofurantoin SK MOH 2013: (MACROBID) remains at 96% or better in Saskatchewan (per recent antibiograms). UTI in Continuing Care Settings “I used to be https://saskpic.ipac-canada.org/ so strong...” STAYING POWER photos/custom/UTI%20Guidelines%20 2) 60% - Are you kidding?! 19April2013.pdf In some institutional settings, like long-term care, E. coli resistance to ciprofloxacin can be as high as ~60%. No wonder antimicrobial SOGC 2010: stewardship messaging suggests “Reserve to Preserve” for when we really Recurrent UTI C IPROFLOXAC I N need it! http://www.jogc.com/article/S1701- 2163(16)34717-X/pdf 3) Urine cultures are not required - for most Skip the Urine Req. -

February 2019 Volume 42 Number 1
February 2019 Volume 42 Number 1 AN INDEPENDENT REVIEW nps.org.au/australian-prescriber CONTENTS EDITORIAL Does size matter? Addressing 2 pack size and antibiotic duration TM McGuire ARTICLES Optimal antimicrobial duration 5 for common bacterial infections HL Wilson, K Daveson, CB Del Mar Prescribing for 10 transgender patients L Tomlins Nitrofurantoin and fosfomycin for 14 resistant urinary tract infections: old drugs for emerging problems BJ Gardiner, AJ Stewardson, IJ Abbott, AY Peleg Prescribing for adolescents 20 M Kang, K Kim The hot patient: acute 24 drug-induced hyperthermia N Jamshidi, A Dawson LETTERS TO THE EDITOR 4 NEW DRUGS 29 Alirocumab for hypercholesterolaemia Apalutamide for prostate cancer Baricitinib for rheumatoid arthritis Migalastat for Fabry syndrome Rufinamide for seizures Tildrakizumab for psoriasis Emicizumab for haemophilia A VOLUME 42 : NUMBER 1 : FEBRUARY 2019 EDITORIAL Does size matter? Addressing pack size and antibiotic duration Treasure M McGuire In Australia, most antibiotics are prescription- therefore be influenced by not only the dose Assistant director of only so prescribers are the custodians of judicious prescribed but also the inherent characteristics of pharmacy, Mater Health use. They need to balance their concerns about the antibiotic.7 Services, Brisbane antibiotic resistance with their responsibility for Conjoint senior lecturer, Judicious antibiotic use needs to balance prescribing individual patient management. Prescribing with no School of Pharmacy, for too short a period (causing treatment failure, University of Queensland, clinical indication, inappropriate drug choice, and delayed return to health or the development of Brisbane suboptimal dosing and duration can all contribute to complications) with overprescribing which increases 1,2 Associate professor of antimicrobial resistance. -

Mechanisms of Cocaine Abuse and Toxicity
Mechanisms of Cocaine Abuse and Toxicity U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES • Public Health Service • Alcohol, Drug Abuse, and Mental Health Administration I Mechanisms of Cocaine Abuse and Toxicity Editors: Doris Clouet, Ph.D. Khursheed Asghar, Ph.D. Roger Brown, Ph.D. Division of Preclinical Research National Institute on Drug Abuse NIDA Research Monograph 88 1988 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Alcohol, Drug Abuse, and Mental Health Administration National Institute on Drug Abuse 5600 Fishers Lane Rockville, MD 20857 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, DC 20402 NIDA Research Monographs are prepared by the research divisions of the National Institute on Drug Abuse and published by its Office of Science. The primary objective of the series is to provide critical reviews of research problem areas and techniques, the content of state-of-the-art conferences, and integrative research reviews. Its dual publication emphasis is rapid and targeted dissemination to the scientific and professional community. Editorial Advisors MARTIN W. ADLER, Ph.D. MARY L. JACOBSON Temple University School of Medicine National Federation of Parents for Philadelphia,Pennsylvania Drug-Free Youth Omaha, Nebraska SYDNEY ARCHER, Ph.D. Rensselaer Polytechnic lnstitute Troy, New York REESE T. JONES, M.D. Langley Porter Neuropsychiatric lnstitute RICHARD E. BELLEVILLE, Ph.D. San Francisco, California NB Associates, Health Sciences RockviIle, Maryland DENISE KANDEL, Ph.D. KARST J. BESTEMAN College of Physicians and Surgeons of Alcohol and Drug Problems Association Columbia University of North America New York, New York Washington, D.C. GILBERT J. -
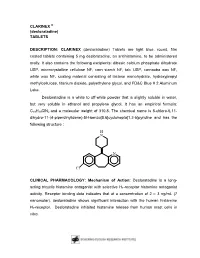
CLARINEX ® (Desloratadine) TABLETS
CLARINEX ® (desloratadine) TABLETS DESCRIPTION: CLARINEX (desloratadine) Tablets are light blue, round, film coated tablets containing 5 mg desloratadine, an antihistamine, to be administered orally. It also contains the following excipients: dibasic calcium phosphate dihydrate USP, microcrystalline cellulose NF, corn starch NF, talc USP, carnauba wax NF, white wax NF, coating material consisting of lactose monohydrate, hydroxypropyl methylcellulose, titanium dioxide, polyethylene glycol, and FD&C Blue # 2 Aluminum Lake. Desloratadine is a white to off-white powder that is slightly soluble in water, but very soluble in ethanol and propylene glycol. It has an empirical formula: C19H19ClN2 and a molecular weight of 310.8. The chemical name is 8-chloro-6,11- dihydro-11-(4-piperdinylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine and has the following structure : H N N Cl CLINICAL PHARMACOLOGY: Mechanism of Action: Desloratadine is a long- acting tricyclic histamine antagonist with selective H1-receptor histamine antagonist activity. Receptor binding data indicates that at a concentration of 2 – 3 ng/mL (7 nanomolar), desloratadine shows significant interaction with the human histamine H1-receptor. Desloratadine inhibited histamine release from human mast cells in vitro. Results of a radiolabeled tissue distribution study in rats and a radioligand H1- receptor binding study in guinea pigs showed that desloratadine did not readily cross the blood brain barrier. Pharmacokinetics: Absorption: Following oral administration of desloratadine 5 mg once daily for 10 days to normal healthy volunteers, the mean time to maximum plasma concentrations (Tmax) occurred at approximately 3 hours post dose and mean steady state peak plasma concentrations (Cmax) and area under the concentration-time curve (AUC) of 4 ng/mL and 56.9 ng⋅hr/mL were observed, respectively.