PHASE EQUILIBRIUM DATA for the SYSTEM Mgo-Mgfz-Sioz Wrr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Abbreviations
List of Abbreviations Ab albite Cbz chabazite Fa fayalite Acm acmite Cc chalcocite Fac ferroactinolite Act actinolite Ccl chrysocolla Fcp ferrocarpholite Adr andradite Ccn cancrinite Fed ferroedenite Agt aegirine-augite Ccp chalcopyrite Flt fluorite Ak akermanite Cel celadonite Fo forsterite Alm almandine Cen clinoenstatite Fpa ferropargasite Aln allanite Cfs clinoferrosilite Fs ferrosilite ( ortho) Als aluminosilicate Chl chlorite Fst fassite Am amphibole Chn chondrodite Fts ferrotscher- An anorthite Chr chromite makite And andalusite Chu clinohumite Gbs gibbsite Anh anhydrite Cld chloritoid Ged gedrite Ank ankerite Cls celestite Gh gehlenite Anl analcite Cp carpholite Gln glaucophane Ann annite Cpx Ca clinopyroxene Glt glauconite Ant anatase Crd cordierite Gn galena Ap apatite ern carnegieite Gp gypsum Apo apophyllite Crn corundum Gr graphite Apy arsenopyrite Crs cristroballite Grs grossular Arf arfvedsonite Cs coesite Grt garnet Arg aragonite Cst cassiterite Gru grunerite Atg antigorite Ctl chrysotile Gt goethite Ath anthophyllite Cum cummingtonite Hbl hornblende Aug augite Cv covellite He hercynite Ax axinite Czo clinozoisite Hd hedenbergite Bhm boehmite Dg diginite Hem hematite Bn bornite Di diopside Hl halite Brc brucite Dia diamond Hs hastingsite Brk brookite Dol dolomite Hu humite Brl beryl Drv dravite Hul heulandite Brt barite Dsp diaspore Hyn haiiyne Bst bustamite Eck eckermannite Ill illite Bt biotite Ed edenite Ilm ilmenite Cal calcite Elb elbaite Jd jadeite Cam Ca clinoamphi- En enstatite ( ortho) Jh johannsenite bole Ep epidote -

Clintonite-Bearing Assemblages in Chondrodite Marbles from the Contact Aureole of the Tøebíè Pluton, Moldanubian Zone, Bohemian Massif
Journal of the Czech Geological Society 51/34(2006) 249 Clintonite-bearing assemblages in chondrodite marbles from the contact aureole of the Tøebíè Pluton, Moldanubian Zone, Bohemian Massif Asociace obsahující clintonit v chondroditových mramorech moldanubika z kontaktní aureoly tøebíèského plutonu, Èeský masiv (6 figs, 4 tabs) STANISLAV HOUZAR1 MILAN NOVÁK2 1 Department of Mineralogy and Petrography, Moravian Museum, Zelný trh 6, CZ-659 37 Brno, Czech Republic; [email protected] 2 Institute of Geological Sciences, Masaryk University, Kotláøská 2, CZ-611 37 Brno, Czech Republic; [email protected] Clintonite is a minor to accessory mineral in chondrodite marbles. They represent a rare type of metacarbonate rocks in the Varied Unit of the Moldanubian Zone, forming thin bodies enclosed in migmatites. Clintonite occurs exclusively in marbles from contact aureole of melanocratic ultrapotassic granites (durbachites) of the Tøebíè Pluton. Chondrodite marbles consist of dominant calcite, less abundant dolomite; amounts of silicates vary from ~ 5 to 30 vol. %. The early mineral assemblage Dol+Cal+Prg ±Phl is replaced by the assemblage Chn+Cli+Cal ±Chl I ±Spl. Accessory minerals include fluorapatite, diopside, tremolite, pyrrhotite, and rare zircon and baddeleyite. Violet fluorite occurs on late fissures. Clintonite forms colourless to pale green flakes and sheaf-like aggregates, up to 2 mm in size. It has extraordinary high Si (2.7392.986 apfu) and Si/Al ratio (0.520.60). The contents of Fe (0.0410.128 apfu), Na (0.0350.134 apfu), tot Ti (0.0040.024 apfu) and K (≤ 0.005 apfu) are low. High concentrations of F (0.4371.022 apfu) corresponding up to 26 % of the F-component are the highest ever-recorded in clintonite. -

Bulletin 65, the Minerals of Franklin and Sterling Hill, New Jersey, 1962
THEMINERALSOF FRANKLINAND STERLINGHILL NEWJERSEY BULLETIN 65 NEW JERSEYGEOLOGICALSURVEY DEPARTMENTOF CONSERVATIONAND ECONOMICDEVELOPMENT NEW JERSEY GEOLOGICAL SURVEY BULLETIN 65 THE MINERALS OF FRANKLIN AND STERLING HILL, NEW JERSEY bY ALBERT S. WILKERSON Professor of Geology Rutgers, The State University of New Jersey STATE OF NEw JERSEY Department of Conservation and Economic Development H. MAT ADAMS, Commissioner Division of Resource Development KE_rr_ H. CR_V_LINCDirector, Bureau of Geology and Topography KEMBLEWIDX_, State Geologist TRENTON, NEW JERSEY --1962-- NEW JERSEY GEOLOGICAL SURVEY NEW JERSEY GEOLOGICAL SURVEY CONTENTS PAGE Introduction ......................................... 5 History of Area ................................... 7 General Geology ................................... 9 Origin of the Ore Deposits .......................... 10 The Rowe Collection ................................ 11 List of 42 Mineral Species and Varieties First Found at Franklin or Sterling Hill .......................... 13 Other Mineral Species and Varieties at Franklin or Sterling Hill ............................................ 14 Tabular Summary of Mineral Discoveries ................. 17 The Luminescent Minerals ............................ 22 Corrections to Franklln-Sterling Hill Mineral List of Dis- credited Species, Incorrect Names, Usages, Spelling and Identification .................................... 23 Description of Minerals: Bementite ......................................... 25 Cahnite .......................................... -

Hydrothermal Crystal Growth of Metal Borates for Optical Applications Carla Heyward Clemson University, [email protected]
Clemson University TigerPrints All Dissertations Dissertations 8-2013 Hydrothermal Crystal Growth of Metal Borates for Optical Applications Carla Heyward Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Part of the Chemistry Commons Recommended Citation Heyward, Carla, "Hydrothermal Crystal Growth of Metal Borates for Optical Applications" (2013). All Dissertations. 1164. https://tigerprints.clemson.edu/all_dissertations/1164 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. HYDROTHERMAL CRYSTAL GROWTH OF METAL BORATES FOR OPTICAL APPLICATIONS A Dissertation Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Chemistry by Carla Charisse Heyward August 2013 Accepted by: Dr. Joseph Kolis, Committee Chair Dr. Shiou-Jyh Hwu Dr. Andrew Tennyson Dr. Gautam Bhattacharyya ABSTRACT Crystals are the heart of the development of advance technology. Their existence is the essential foundation in the electronic field and without it there would be little to no progress in a variety of industries including the military, medical, and technology fields. The discovery of a variety of new materials with unique properties has contributed significantly to the rapidly advancing solid state laser field. Progress in the crystal growth methods has allowed the growth of crystals once plagued by difficulties as well as the growth of materials that generate coherent light in spectral regions where efficient laser sources are unavailable. The collaborative progress warrants the growth of new materials for new applications in the deep UV region. -

THE FRANKLIN STERLING MINERAL AREA by Helen A~ Biren, Brooklyn College " TRIP
'-_.-. E-l THE FRANKLIN STERLING MINERAL AREA by Helen A~ Biren, Brooklyn College " TRIP, . E Introduction The area which we shall visit is 'a limestone region lying in the New Jersey Highlands, which is part of the Reading Prong. It extends in a northeasterly direction ac~os5 the northern part of the state. The rocks are Precamqrian "crystal,lines" with narrow,beltsof in . folded and infaul ted Paleozoic. sedimentary rocks. Major 10ngi.::tr,~9;nal faults slice the fold structures,so that the area has been ,described as a series of fault blcicks e~iendingfr6m south of tbe Sterling Mine to Big Island, N. Y. ", ", For. many years the Franklin Limeston~iyielded enough zinc .to make 'New Jersey a, leading producer of this commo~!~ty.~)',Mining has steadily de creased in thi'sarea, and in '1955 thefrankl.inMine ~as shut dm.W1 perman :eDtl y, so .that mineral specimens are4eri ve({:ma~~l y ,from surfqce dumps and quarries. Some twenty million tons of ore were remQved from Franklin before it was shut down. Prior to mining, the ore outcropped in two synclinal folds com pletely within the limestone, which pitched to the northeast at an angle .. _" ,of about 250 with the horizontal. In these two horseshoe shaped bodies "Were developed the Franklin and the Ste,r,Ung M~nes. This zinc ore :h,,: unique in its lack of sulfides and lead mir,tera~~:, and in the occurrence 'of frankliniteand zincite as substantia~~ore m~nerals. : :: The limestone has produced nearly 200 species of minerals, some 33 of which were first found ip,Fr(inklin,and .about 30 of which have never been found el$emere. -

First Annual Mineralogy Exhibit of the Franklin Kiwanis Club
FIRST ANNUAL MINERALCGY EXHIBIT CF THE FRANKLIN KIWANIS CLUB - October 26th & 27th, 1957 This exhibit makes available for public view for the first time many of the principal private collections of the unique minerals of the Franklin-Sterling Area. These unusual ore bodies, comprising more minerals than are found anywhere else on earth, have intrigued mineralogists and collectors ever since their discovery. Dutch mining experts first explored the area in 1640. The ore bodies, whose principal metals are zinc, manganese and iron, continued to puzzle experts for the next two centuries. Mining operations were first successfully developed by The New Jersey Zinc Company, which succeeded in building a great industry from its beginnings at Franklin. The deposits at Franklin are now exhausted and its specimens have become collectors items. The 178 minerals found at Franklin-Sterling and the 29 minerals which have never been found elsewhere are listed below. MINERALS OF THE FRANKLIN-STERLING AREA Agurite Calcium, Lar- Halloysite Nasonite Albite senite Hancockite Ne oto c it e Allactite Celestite Hardystonite Niccolite Allanite Cerusite Hedyphane. Norbergite Amphibole Chalcocite Hematite Oligocase Actinolite Chalcophanite Hetacrolite Pararammels- Crocidolite Chalcopyrite Heulandite. ' bergite Cummingtonite Chleanthite Hodgkinsonite Pectolite Edenite Chlorite Holdenite Phlogopite Hastingsite Chlorophoenicite Hortonolite Prehnite Hornblende Magnesium Chl. Hyalophane Psilomelane Pargasite Chondrocite Hydrohans- Pyrite Tremolite Clinohedrite mannite -

The Picking Table Volume 46 – Spring/Fall 2005
THE PICKING TABLE JOURNAL OF THE FRANKLIN-OGDENSBURG MINERALOGICAL SOCIETY VOL. 46 — SPRING/FALL 2005 $20.00 U.S. INSIDE THIS ISSUE: • The geology of Double Rock pegmatite • The Kemble family, long-term Franklin mineral collectors • Grunerite added to the Franklin-Sterling Hill species list • More reflections on Franklin wollastonite The contents of The Picking Table are licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. The Franklin-Ogdensburg Mineralogical Society, Inc. Officers, 2005-2007 PRESIDENT ASSISTANTTREASURER LIAISON WITH THE EASTERN COMMITTEE CHAIRPERSONS FEDERATION OF MINERAL FREDYOUNG WILLIAM KROTH AND LAPIDARY SOCIETIES Auditing 234 Warbasse Junction Road 240 Union Avenue (EFMLS) WILLIAM J.TROST Field Trip Lafayette NJ 07848 Wood-Ridge NJ 07075 Delegate EDWARD H.WILK (973)579-1575 (201)933-3029 JOE KAISER WARREN CUMMINGS Alternate Historical VICE-PRESIDENT SLIDE COLLECTION RICHARD C. BOSTWICK CUSTODIAN JOHNL.BAUM GREG JACOBUS Identification — 36 Post Blvd. EDWARD H.WILK Mineral Exchange Carteret NJ 07008 202 Boiling Springs Avenue RICHARD C. BOSTWICK (732) 829-2869 E. Rutherford NJ 07073 Nominating Program WILLIAM KROTH [email protected] (201)438-8471 Spring Swap & Sell CHESTER S. LEMANSKI, JR. SECOND VICE-PRESIDENT TRUSTEES MARK BOYER C. RICHARD BIELING (2005-2007) 25 Cork Hill Road RICHARD C.BOSTWICK Ogdensburg NJ 07439 MEMBERSHIP INFORMATION (2004-2006) (973)209-8319 JOHNC.EBNER Anyone interested in the minerals, mines, or mining history of [email protected] the Franklin-Ogdensburg, New Jersey, area is invited to join (2004-2006) the Franklin-Ogdensburg Mineralogical Society, Inc. (FOMS). GEORGE ELLING Membership includes scheduled meetings, lectures, and field SECRETARY (2005-2007) trips, as well as a subscription to The Picking Table. -

NEW Daila on the STRUCTURE of Norbergffe: LOCATION OF
1523 The Canadian M ineralo gi st Vol.35,pp. 1523-1s30(197) NEW DAilA ON THESTRUCTURE OF NORBERGffE:LOCATION OF HYDROGEN BVX.RAY DIFFRACTION FERNANDOCAMARAT CNR- Centrodi Sndiaper la Cristallpchimicae Ia Cristallografia(CSCC), via Abbiategrasso 209' I-27100 Pavta ltaly ABSTRACT T\wo crystals of OH-rich norbergite from the Alpujarride Complex @etic Cordilleras) have been analyzed, and their structue rfinedby single-crystalX-raydiftaction analysis.Tbe new dataconfirm the earlierrefinementon a comlnsition near the F end-membr, and add new insight to the crystal chemistry of this humite-groupmineral. In particular, hydrogen atoms were locatedon the difference-Fouriermap. l1eir position and the consequentstuctural constraintsto the (OH)F-1substiortion suggestthat OH-rich norbergite requirei very high pressureto crystallize, thus expLiriningthe normal occurrenceof the fluorine-dominant compositions. Keywords: norbergite,humite group, structurerefinement, electon-microprobe data,hydrogen. Sotffens Deux cristauxde norbergiterelativenent riches en oII, provenantdu complexede Alpujauide' dansles cordilldre'sbetiques d'Espague,ont 6t6 analys6s,et lur stuctre a 6td affin6epar diftaction X sur cristal unique.Les donn€esnouvelles confirment les rdsultatsde I'affinement ant6rieur,obtenu sur un cristal prochedu p0le fluo€, et ajoutentdes nouveaux renseiguements au sujet de la cristallochimiede ce membredu group de la humite. En particulier, les atomesd'hydrog0ne ont 6td repdr€ssur une projection de Fourier par diff6rence, D'aprds leur position et les contraintes structurales qui en d&oulent concernant la substitution (OII)F-', la formation de la norbergiteriche en OH requiert une pressionassez 6levde, ce qui rend comptede la pr6sencecourante de compositionsi dominanceder fluor. (fraduit par la R6daction) Mots-cMs:norbergite, groupe de la humite, affinement de la structure,donn6es de microsonde6lectotrique, hydrogine. -
Crystal Chemistry of the Humite Minerals
CRYSTAL CHEMISTRY OF THE HUMITE MINERALS by Norris William Jones Thesis submitted to the Graduate Faculty of the Virginia Polytechnic Institute in partial fulfillment for the degree of DOCTOR OF PHILOSOPHY in Geological Sciences APPROVED: Chairman, P. H. Ribbe G. V. Gibbs R. V. Dietrich "T W. D. Lo-.;:;y J. W. Murray June, 1968 Blacksburg, Virginia TABLE OF CONTENTS Page ·Acknowledgements . •• iv List of Tables . v List of Figures • . • •• vi INTRODUCTION . • . • . 1 CRYSTAL STRUCTURES OF THE HUMITES . 4 General . • . • . 4 Comparison of the humite minerals and olivine . 9 Morphotropy . •• 16 Epitaxial intergrowths and twinning . •• 17 Choice of space group and crystallographic axes • 20 CHEMISTRY OF THE HUMITE MINERALS •• . •• 24 Composition . .. •• 24 Compositional variation . • 29 Stoichiometric considerations . • • 32 Electron microprobe analyses . • 38 RELATIONSHIP BETWEEN COMPOSITION AND CELL PARAMETERS •• • 49 ' . (Fe + Mn) for Mg. • • • • • • • • • • • • • • • • . • • 49 Ti for Mg •••• . • • • 52 (OH,F) for 0 . • 52 SUMMARY AND CONCLUSIONS . • •• 55 APPENDIX A: Chemical analyses of the humite minerals • • • 57 APPENDIX B: Calculations of OH, -0, and stoichiometric ratios • 72 APPENDIX C: Microprobe techniques • ~ • • • • • • • • • • • • • 75 ii iii Page Operating conditions • • • • • • • • • • • • • • 76 Data corrections • • • • • • • • • • • • • • • • • 77 Drift. • • • • • • • • • • • • • 80 Dead time. • • • • • • • • • • • • • • • • 80 Background . • • • • • • • • • • • . 81 Mass absorption. • • • • • • • • . 81 Atomic number. -
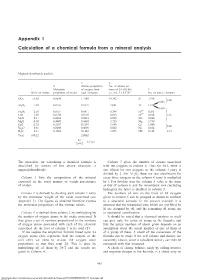
Appendix 1 Calculation of a Chemical Formula from a Mineral Analysis
Appendix 1 Calculation of a chemical formula from a mineral analysis Appendix 1 Magnesiohornblende analysis 3 4 2 Atomic proportion No. of anions on 1 Molecular of oxygen from basis of 24 (O,OH) 5 Wt.% of oxides proportion of oxides each molecule i.e. col. 368.3735 No. of ions in formula SiO 51.63 0.8594 1.7188 14.392 Si 7.196 2 8.00 0.804 } Al2O3 7.39 0.0725 0.2175 1.821 Al 1.214 0.410 3+ Fe2O3 2.50 0.0157 0.0471 0.394 Fe 0.263 FeO 5.30 0.0738 0.0738 0.618 Fe2+ 0.618 5.07 MnO 0.17 0.0024 0.0024 0.020 Mn 0.020 } MgO 18.09 0.4489 0.4489 3.759 Mg 3.759 CaO 12.32 0.2197 0.2197 1.840 Ca 1.840 2.00 Na2O 0.61 0.0098 0.0098 0.082 Na 0.164 } H2O+ 2.31 0.1282 0.1282 1.073 OH 2.146 2.15 Total 100.32 2.8662 24 = 8.3735 2.8662 The procedure for calculating a chemical formula is Column 5 gives the number of cations associated described by means of the above example, a with the oxygens in column 4. Thus for SiO2 there is magnesiohornblende. one silicon for two oxygens so the column 4 entry is divided by 2. For A12O3 there are two aluminiums for Column 1 lists the composition of the mineral every three oxygens so the column 4 entry is multiplied expressed in the usual manner as weight percentages by ~˜. -

The Compressibility of Natural Norbergite, Mg2.98Fe0.01Ti0.01Si0
Photon Factory Activity Report 2004 #22 Part B (2005) Crystallography 10A/2003G208 The compressibility of natural norbergite, Mg2.98Fe0.01Ti0.01Si0.99O4(OH0.31, F1.69) Takahiro KURIBAYASHI1*, Masahiko TANAKA2,3, Yasuhiro KUDOH1 1Institute of Mineralogy, Petrology and Economic Geology, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan 3WEBRAM, MIMS, Nishi-Harima, Hyogo 679-5198, Japan Introduction In similarly, the isothermal bulk modulus of this Norbergite (n=1), which is a hydrous magnesium specimen was calculated as K=113(2) GPa assuming K′=4. silicate mineral, is known as one of the humite minerals This result is plotted on the simple positive correlation [3] ⋅ ρ described as nMg2SiO4 Mg(OH,F)2 (n=1-4). Also, between K and density ( ) of the minerals in some dense forsterite, Mg2SiO4, is known as the most important hydrous silicate minerals (Fig. 1). mineral in the Earth′s mantle. Forsterite and the humite minerals constitute the polysomatic series. The humite Table 1. The lattice parameters of natural norbergite minerals are important in the view of the carriers and at each pressure point up to 8.2 GPa reservoirs of water in the Earth′s deep interior, because P (GPa) ab c V these minerals are stable under high-pressure and high- 0.0001 4.709(2) 10.279(3) 8.754(4) 423.7(2) temperature mantle conditions [1]. 0.5 4.701(2) 10.254(2) 8.736(3) 421.1(2) We performed a high-pressure single crystal X-ray 3.1 4.674(2) 10.176(2) 8.670(3) 412.3(2) diffraction study on a natural norbergite at ambient, 0.5, 4.2 4.662(2) 10.138(2) 8.642(3) 408.5(2) 3.1, 4.2, 4.7, 5.4, 6.3, 7.6 and 8.2 GPa in order to 4.7 4.657(2) 10.120(3) 8.626(3) 406.5(2) investigate its compressibility and crystal structure. -

The Picking Table Volume 1, No. 2
THE PICKING T^BLE FRfiNKLIN-GGDENSBURG hINERaLOGICAL bOCISTY, BOX 146 FRANKLIN, NEW JERSEY Volume 1 JUNE, I960 Number 2 The contents of The Picking Table are licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. CONTACT - KTiiliORrHIC MIJJEELilS - A Resume Professor .Arthur Montgomery, Lafayette College, Easton, Penna. Metamorphism is a changing form, the formation of new minerals and structures caused by different conditions of temperature, pressure and/or solutions. If pressure aloneis involved, the metamorphism takes place near the earth's surface. There is no recrystallization, but banded schistose rocks result. When heat and pressure are combined, the reactions occur in the depths and regional metamorphism takes place. Shaly rocks are converted to schist and limestone to marble. The minerals formed are usually parallel, platy and blade-like as in gneisses; layers or bands of aggregates of such minerals typify gneisses. Franklin ore is banded; gneiss is common in the footwall. Regional metamorphism is probably one of the first steps that occurred at Franklin. where heat is the most important factor, the transformation is called contact-metamorphism. This usually involves contact between igneous siliceous intrusive material and limestone host rock. In its simplest form, thermal contact-metamorphism, this occurs in the absence of solutions. Since directed pressure is not a factor, there is no parallel orientation of minerals. If solutions are important, as when granite is involved and boron, water and halides are available, the process is called hydrothermal contact-metamorphism, or metasomism, and a wide variety of minerals may be fomred. The number of minerals is enhanced if the host limestone is dolomitic, as it is in part at Franklin, and thus releases magnesium as well as calcium to react with the intrusive rock.