Flavor Profiles of Monovarietal Virgin Olive Oils Produced in the Oriental
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

& Special Prizes
Αthena International Olive Oil Competition 26 ΧΑΛΚΙΝΑ- 28 March ΜΕΤΑΛΛΙΑ* 2018 OLIVE OIL PRODUCER DELPHIVARIETAL MAKE-UP• PHOCIS REGION COUNTRY WEBSITE ΜEDALS & SPECIAL PRIZES Final Participation and Awards Results For its third edition the Athena International Olive Oil Competition (ATHIOOC) showed a 22% increase in the num- ber of participating samples; 359 extra virgin olive oils from 12 countries were judged by a panel of 20 interna- tional experts from 11 countries. This is the first year that the number of samples from abroad overpassed those from Greece: of the 359 samples tasted, 171 were Greek (48%) and 188 (52%) from other countries. In conjunction with the high number of inter- national judges (2/3 of the tasting panel), this establishes Athena as one of the few truly international extra virgin olive oil competitions in the world ―and one of the fastest growing ones. ATHIOOC 2018 awarded 242 medals in the following categories: 13 Double Gold (scoring 95-100%), 100 Gold (scoring 85-95%), 89 Silver (scoring 75-85%) and 40 Bronze (scoring 65-75%). There were also several special prizes including «Best of Show» which this year goes to Palacio de los Olivos from Andalusia, Spain. There is also a notable increase in the number of cultivars present: 124 this year compared to 92 last year, testify- ing to the amazing diversity of the olive oil world. The awards ceremony will take place in Athens on Saturday, April 28 2018, 18:00, at the Zappeion Megaron Con- ference & Exhibitions Hall in the city center. This event will be preceded by a day-long, stand-up and self-pour tasting of all award-winning olive oils. -

Publication of an Amendment Application Pursuant to Article 6(2) of Council Regulation (EC) No 510/2006 on the Protection Of
C 186/18 EN Official Journal of the European Union 26.6.2012 Publication of an amendment application pursuant to Article 6(2) of Council Regulation (EC) No 510/2006 on the protection of geographical indications and designations of origin for agricultural products and foodstuffs (2012/C 186/10) This publication confers the right to object to the amendment application pursuant to Article 7 of Council Regulation (EC) No 510/2006 ( 1). Statements of objection must reach the Commission within six months of the date of this publication. AMENDMENT APPLICATION COUNCIL REGULATION (EC) No 510/2006 AMENDMENT APPLICATION IN ACCORDANCE WITH ARTICLE 9 ‘ΚΑΛΑΜΑΤΑ’ (KALAMATA) EC No: EL-PDO-0117-0037-21.12.2009 PGI ( ) PDO ( X ) 1. Heading in the specification affected by the amendment: — Name of product — ☒ Description of product — ☒ Geographical area — Proof of origin — ☒ Method of production — ☒ Link — Labelling — National requirements — Other (please specify) 2. Type of amendment(s): — Amendment to single document or summary sheet — ☒ Amendment to specification of registered PDO or PGI for which neither the single document nor the summary sheet has been published — Amendment to specification that requires no amendment to the published single document (Article 9(3) of Regulation (EC) No 510/2006) — Temporary amendment to specification resulting from imposition of obligatory sanitary or phytosanitary measures by public authorities (Article 9(4) of Regulation (EC) No 510/2006) 3. Amendment(s): 3.1. Description of product: In this application the olive oil produced is described in greater detail than in the initial registration dossier. Stricter quality specifications are laid down in order to ensure that the name is used only for the area's very best quality olive oil. -

Assessment of Maternal Effects and Genetic Variability in Resistance to Verticillium Dahliae in Olive Progenies
Article Assessment of Maternal Effects and Genetic Variability in Resistance to Verticillium dahliae in Olive Progenies Pedro Valverde Caballero, Carlos Trapero Ramírez, Diego Barranco Navero, Francisco J. López-Escudero, Ana Gordon Bermúdez-Coronel and Concepción Muñoz Díez * Department of Agronomy (Excellence Unit ‘María de Maeztu’ 2020-23), ETSIAM, University of Córdoba, 14071 Córdoba, Spain; [email protected] (P.V.C.); [email protected] (C.T.R.); [email protected] (D.B.N.); [email protected] (F.J.L.-E.); [email protected] (A.G.B.-C.) * Correspondence: [email protected] Abstract: The use of genetic resistance is likely the most efficient, economically convenient and en- vironmentally friendly control method for plant diseases, as well as a fundamental piece in an inte- grated management strategy. This is particularly important for woody crops affected by diseases in which mainly horizontal resistance mechanisms are operative, such as Verticillium wilt, caused by Verticillium dahliae. In this study, we analyzed the variability in resistance to Verticillium wilt of olive trees in progenies from five crosses: ‘Picual’ × ‘Frantoio’, ‘Arbosana’ × ‘Koroneiki’, ‘Sikitita’ × Citation: Valverde, P.; Trapero, C.; ‘Arbosana’, ‘Arbosana’ × ‘Frantoio’ and ‘Arbosana’ × ‘Arbequina’ and their respective reciprocal Barranco, D.; López-Escudero, F.J.; crosses. Additionally, seedlings of ‘Picual’ and ‘Frantoio’ in open pollination were used as controls. Gordon, A.; Díez C. M. Assessment In October 2016 and 2018, the fruits were harvested, and seeds germinated. Six-week-old seedlings of maternal effect and genetic varia- were inoculated by dipping their bare roots in a conidial suspension of V. dahliae, and disease pro- bility in resistance to Verticillium gress in terms of symptom severity and mortality was evaluated weekly. -

Kreta Reserve Extra Virgin Olive Oil Information and Retail Pricing
Kreta Reserve Extra Virgin Olive Oil Information and Retail Pricing You cannot buy a higher quality or more healthful extra virgin olive oil at any price! Grown, Harvested, Pressed, Packed, and Imported by GMHS International, LLC - Tony Sansone, CEO Phone: 850-368-7162 Email: [email protected] www.kretareserve.com + Super Low Acidity: 0.22% The acidity in extra virgin olive oil is the first and foremost indicator of quality. Each crop year, we strive to produce oil with the lowest possible acidity level. The best oils we can find on the market have acidity levels of 0.3% or higher. Per standards set by the International Olive Oil Council, extra virgin olive oil can have acidity as high as 0.8%. Thus, Kreta Reserve has achieved a unique level of quality. + Super Low Peroxide Value: 9 meq O2 /Kg In simple terms, the peroxide value of an extra virgin olive oil tells us how long the oil will last before it turns rancid. It is an indicator of the quantity of particles in the oil that have oxidized. For example, if you allow damaged, rotten, or rancid olives to be processed along with good olives, then the peroxide val- ue will be high. The International Olive Oil Council allows a peroxide value of 20 or less for extra virgin olive oil. Our 2009 harvest crop had a very low peroxide value of 9. Kreta Reserve EVOO will remain fresh and wholesome for years when stored properly. In the better olive oils we tested, the peroxide value range was 12-14. -
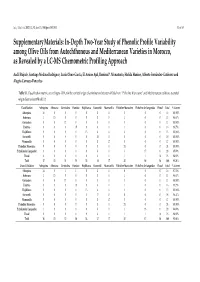
In-Depth Two-Year Study of Phenolic Profile Variability Among
Int. J. Mol. Sci. 2017, 18, 52; doi:10.3390/ijms18010052 S1 of S3 Supplementary Materials: In-Depth Two-Year Study of Phenolic Profile Variability among Olive Oils from Autochthonous and Mediterranean Varieties in Morocco, as Revealed by a LC-MS Chemometric Profiling Approach Aadil Bajoub, Santiago Medina-Rodríguez, Lucía Olmo-García, El Amine Ajal, Romina P. Monasterio, Hafida Hanine, Alberto Fernández-Gutiérrez and Alegría Carrasco-Pancorbo Table S1. Classification matrix, according to LDA, for the varietal origin discrimination between VOOs from “Picholine Marocaine” and Mediterranean cultivars (varietal origin discriminant Model 1). Classification Arbequina Arbosana Cornicabra Frantoio Hojiblanca Koroneiki Manzanilla Picholine Marocaine Picholine de Languedoc Picual Total % Correct Arbequina 16 0 0 0 0 0 0 0 0 0 16 100.00% Arbosana 1 13 0 0 0 0 0 1 0 0 15 86.67% Cornicabra 0 0 11 0 0 0 0 0 0 0 11 100.00% Frantoio 0 0 0 15 0 0 0 1 0 0 16 93.75% Hojiblanca 0 0 0 0 13 0 0 0 0 0 13 100.00% Koroneiki 0 0 0 0 0 18 0 0 0 0 18 100.00% Manzanilla 0 0 0 0 0 0 17 0 0 0 17 100.00% Picholine Marocaine 0 0 0 0 0 0 0 24 0 0 24 100.00% Picholine de Languedoc 0 0 0 0 0 0 0 3 17 0 20 85.00% Picual 0 0 0 0 0 0 0 1 1 16 18 88.89% Total 17 13 11 15 13 18 17 30 18 16 168 95.24% Cross-Validation Arbequina Arbosana Cornicabra Frantoio Hojiblanca Koroneiki Manzanilla Picholine Marocaine Picholine de Languedoc Picual Total % Correct Arbequina 14 0 1 1 0 0 0 0 0 0 16 87.50% Arbosana 1 13 0 0 0 0 0 1 0 0 15 86.67% Cornicabra 0 0 11 0 0 0 0 0 0 0 11 100.00% Frantoio 0 0 0 15 0 0 0 1 0 0 16 93.75% Hojiblanca 0 0 0 0 13 0 0 0 0 0 13 100.00% Koroneiki 0 0 0 0 1 17 0 0 0 0 18 94.44% Manzanilla 0 0 0 0 0 0 17 0 0 0 17 100.00% Picholine Marocaine 0 0 0 0 0 0 0 24 0 0 24 100.00% Picholine de Languedoc 1 0 0 0 0 0 0 3 16 0 20 80.00% Picual 0 0 0 0 0 0 0 1 1 16 18 88.89% Total 16 13 12 16 14 17 17 30 17 16 168 92.86% Int. -

Results ATHENA 2019
Αthena InternationalΧΑΛΚΙΝΑ ΜΕΤΑΛΛΙΑ*Olive Oil Competition OLIVE OIL PRODUCER VARIETAL MAKE-UP COUNTRY NAFPLIONREGION PROVINCE WEBSITE FLAVOURED ΒΙΟ 18–20 March 2019 ΜEDALS & SPECIAL PRIZES Final Participation and Awards Results DOUBLE GOLD 2019 DOUBLE GOLD MEDALS OLIVE OIL PRODUCER VARIETAL MAKE-UP COUNTRY REGION PROVINCE WEBSITE FLAVOURED ΒΙΟ One & Olive One & Olive Koroneiki Greece Peloponnese, Messinia Manesi www.oneolive.gr No Conde de Mirasol Aceites Mirasol Hojiblanca Spain Andalusia Córdoba www.condedemirasol.com No Palacio de Los Olivos Olivapalacios Picual Spain Castilla-La Mancha Ciudad Real www.olivapalacios.es No 60% Picual, Oro Del Desierto Coupage Rafael Alonso Aguilera Spain Andalusia Almeria www.orodeldesierto.com Yes 40% Hojiblanca Picualia Picualia Picual Spain Andalusia Jaén www.picualia.com No Horta Real Olive Gallery Picual Spain Castilla-La Mancha Toledo www.olivegallery.es No Aprutino Pescarese San- Azienda Agricola Sandro 90% Dritta, Italy Abruzzo Pescara No dro di Giacomo di Giacomo 10% Intosso 80% Hojiblanco, Venta del Barón Muela Olives Spain Andalusia Córdoba www.mueloliva.es No 20% Picudo GOLD 2019 GOLD MEDALS OLIVE OIL PRODUCER VARIETAL MAKE-UP COUNTRY REGION PROVINCE WEBSITE FLAVOURED ΒΙΟ Valdenvero Hojiblanco Colival Hojiblanca Spain Castilla-La Mancha Ciudad Real www.colival.com No Hispasur Gold Knolive Oils Picual Spain Andalusia Córdoba www.knolive.com No Aceitera Peninsular 50% Picuda, Olíria Coupage Spain Andalusia Córdoba www.aceiterapeninsular.com No Española 50% Hojiblanca Safir Basil Herbes de -

Effect of Mechanically Harvested Olive Storage Temperature and Duration
et al., 2010; Kalua et al., 2008; Effect of Mechanically Harvested Olive Storage Kiritsakis et al., 1998; Yousfi et al., Temperature and Duration on Oil Quality 2009). Moreover, there is generally no indication of whether the fruit originated from rain-fed or irrigated Arnon Dag1, Smadar Boim, Yulya Sobotin, and Isaac Zipori orchards (Agar et al., 1998; Clodoveo et al., 2007; Dourtoglou et al., 2006; Garcı´a et al., 1996; Inarejos-Garcı´a ADDITIONAL INDEX WORDS. virgin olive oil, temperature, irrigation, mechanized harvest, polyphenols, free fatty acids, peroxide value et al., 2010; Kalua et al., 2008; Kiritsakis et al., 1998; Yousfi et al., SUMMARY. Most newly planted olive (Olea europaea L.) orchards are irrigated and 2009; Youssef et al., 2011), although harvested mechanically. We assessed the effects of olive storage temperature and we may speculate that those which are duration on the resultant oil’s quality in three cultivars from modern orchards. Oil not indicated originated from rain-fed acidity increased with storage temperature and time, most markedly in ‘Barnea’ and orchards. There are almost no such least in ‘Koroneiki’. In ‘Koroneiki’, after 9 days in cool storage (4 and 10 °C), free fatty acid (FFA) level remained constant. Polyphenol (PP) content behaved studies of fruit originating from mod- differently among cultivars: in ‘Picual’, it was relatively invariable; in ‘Barnea’, it ern, irrigated, and mechanically har- decreased moderately; and in ‘Koroneiki’, it decreased sharply to half of its initial vested orchards, although these are value in 4 °C storage and one-sixth its initial value in room temperature storage after becoming more and more common 23 days. -

Future of Texas Olives AOOPA
THE FUTURE OF OLIVES IN TEXAS • Monte Nesbitt OLIVES ARE SOIL, DROUGHT AND SALT TOLERANT AND WE HAVE AN ABUNDANCE OF THOSE CHALLENGES IN TEXAS IS THERE AN OLIVE HISTORY IN TEXAS? Illustration by Silia Goetz, wsj.com KNOWN OLIVE HISTORY-TEXAS • Catholic archives in San Antonio indicate Spanish missionaries did not plant olives in Texas as they did in California (Denney, 1982). • Onderdonk (1900), important Texas nurseryman and fruit explorer wrote favorably of olives in San Luis Potosi, Mexico, but was otherwise silent about them. • Old trees dated 1920’s are reported to exist and bear fruit at places like Asherton & Galveston. • Mortensen (1938) described olive varieties introduced from California as “fair” for Wintergarden area and better for dooryard than commercial purposes. • Hartmann (1951) “Twenty-year effort in South Texas has failed to see fruiting” (Weslaco/Brownsville). Ernest Mortensen, Winterhaven, TX Exp. Station OLIVES ON TEXAS A&M CAMPUS 100’s of Manzanilla trees planted in 1974. Produced fruit in 1977, but were damaged by freezes in ‘78, ‘80, ‘81 (Denney, 1982) Thermal constraints on the productivity of olive (Olea europea L.) in the climates of olive-producing regions and of Texas, Thesis, Texas A&M Dept. of Horticultural Sciences, 1982 Dr. James Denney- deceased PUBLICATIONS • Denney, J.O. and G.R. McEachern. 1983. An analysis of several climatic temperature variables dealing with olive reproduction. J. Amer. Soc. Hort. Sci. 108:578-581. • Denney, J.O., McEachern, G.R. and J.F. Griffiths. 1985. Modeling the thermal adaptability of the olive (Olea europaea L.) in Texas. Agric. For. Meteorol. -

Awards by Producer Los Angeles Extra Virgin Olive Oil Awards
Los Angeles Extra Virgin Olive Oil Awards Awards by Producer 1492 www.Quepu.cl BEST OF CLASS, GOLD MEDAL Robust, Picual, Region Del Maule BEST OF CLASS, GOLD MEDAL Robust, Frantoio, Region Del Maule GOLD MEDAL Delicate, Arbequina, Region Del Maule 1st Origin www.1st-Olive.com GOLD MEDAL Medium, Shodoshima 2015 8 Olivos www.DonRafael.cl BRONZE MEDAL Medium, Lontue Valley Acaia www.Hae-gr.com BRONZE MEDAL Medium, Kolovi, Lesvos Adon and Myrrh www.AdonandMyrrh.com BRONZE MEDAL Medium, Baladi, Lebanon Agropromex www.AgroPromex.com SILVER MEDAL Robust, La Roda de Andalucia 2015 Agura www.AoveAgura.com GOLD MEDAL Medium, Picual, Andalucia Silver - Color & Type - BRONZE MEDAL Medium, Coupage, Andalucia Albares www.JalonMoncayo.com BRONZE MEDAL Medium, One, Moncayo Bronze - Contemporary - Albea Blanca Extra Virgin Olive Oil Collection www.AlbeaBlanca.es SILVER MEDAL Robust, Hojiblanca, Cordoba Gold - Series - SILVER MEDAL Medium, Manzanilla Cacerena Caceres Gold - Series - SILVER MEDAL Medium, Koroneiki, Toledo Gold - Series - Alonso Olive Oil www.AlonsOliveOil.com BEST OF SHOW - DELICATE, BEST OF CLASS, GOLD MEDAL Delicate, Koroneiki, La Estrella BEST OF CLASS, GOLD MEDAL Medium, Ultra Premium, La Estrella GOLD MEDAL Robust, Picual, La Estrella SILVER MEDAL Delicate, Frantoio, La Estrella Bronze - Color & Type - SILVER MEDAL Medium, Coratina, La Estrella Los Angeles Extra Virgin Olive Oil Awards Awards by Producer ALTO Olives www.Alto-Olives.com.au GOLD MEDAL Medium, ROBUST, Abercrombie Wilderness, Southern Tablelands 2016 Altomena www.Altomena.it -

Evaluation of Fatty Acid and Sterol Profiles, California Olive Oil, 2016/17 Season
Evaluation of Fatty Acid and Sterol Profiles, California Olive Oil, 2016/17 Season Evaluation of Fatty Acid and Sterol Profiles California Olive Oil 2016/17 Season Submitted to the Olive Oil Commission of California June 2017 Evaluation of Fatty Acid and Sterol Profiles, California Olive Oil, 2016/17 Season Evaluation of Fatty Acid and Sterol Profiles, California Olive Oil, 2016/17 Season SUMMARY At the request of the Olive Oil Commission of California (OOCC), the UC Davis Olive Center collected California olive oil samples produced in the 2016/17 Season and analyzed fatty acid and sterol profiles. The study team collected 70 single-variety samples of olive oil from California commercial producers. Samples that were found to be outside one or more parameters at the UC Davis laboratory were sent to Modern Olives Laboratory (Woodland, CA) for retesting. Both laboratories agreed that 61 of 70 samples (87 percent) were within the fatty acid and sterol parameters required in California. Nine samples (13 percent) were outside at least one fatty acid or sterol parameter. The Commission may wish to recommend modifications to California olive oil standards so that fatty acid and sterol profile standards accommodate all olive oil produced in California and assess new and advanced methods to analyze olive oil purity with the potential to cost less, be more accurate, and minimize laboratory variability. BACKGROUND The Olive Oil Commission of California requested the UC Davis Olive Center to collect data on the fatty acid and sterol profile of California olive oils from commercial samples. The Commission requested that the Olive Center collect at least 70 samples from a wide range of varieties and counties. -

Preliminary Evaluation of Olive (Olea Europaea L.) Cultivars Under Hot
1 Preliminary Evaluation of Olive (Olea europaea 2 L.) Cultivars Under Hot and Arid Environment of 3 Mexico 4 5 Raúl Leonel Grijalva-Contreras1*, Rubén Macías-Duarte1, Arturo López- 6 Carvajal1, Fabián Robles-Contreras1, Manuel de Jesús Valenzuela-Ruiz1 7 and Fidel Núñez-Ramirez2 8 9 10 11 1* National Research Institute for Forestry, Agriculture and Livestock (INIFAP) Experimental 12 Station of the Coast of Hermosillo. Pascual Encinas Félix No. 21. Colonia la Manga. Postal 13 Code 83220. Hermosillo, Sonora México. 14 2Science Agriculture Institute, Autonomous of Baja California University (ICA-UABC). 15 Carretera Delta s/n Ejido Nuevo León. Postal Code 21705. Baja California, México. 16 17 1918 20 . 21 ABSTRACT 22 Currently in Mexico there are few studies on agronomic management in olive production. The objective of this experiment was to evaluate eleven olive cultivars for table and oil production (Arbequina, Koroneiki, Arbosana, Kalamata, Barnea, Pendolino, Empeltre, Manzanilla of Sevilla, Carboncella, Frantoio and Cassaliva) under hot and arid environment of Mexico. The experiment was carried out during two consecutive years in 2015 and 2016 at National Research Institute for Forestry, Agriculture and Livestock (INIFAP) in the Experimental Station of Caborca, Sonora, Mexico. The plantation was done on March, 2012 using a density of 100 trees ha-1 (10 x 10 m) under drip irrigation system. The parameters evaluated were vegetative parameters, yield, fruit quality and oil content. The experiment was analyzed using a randomized complete block design and five replications. The results showed statistical differences for all parameters evaluated. Arbequina obtained the highest olive yield with 34.5 and 70.3 kg per tree for the first and second year production, respectively and Barnea recorded the highest oil content with 19.2%. -

Oil Fatty Acid Composition of Eighteen Mediterranean Olive Varieties Cultivated Under the Arid Conditions of Boughrara (Southern Tunisia)
GRASAS Y ACEITES, 60 (5), OCTUBRE-DICIEMBRE, 498-506, 2009, ISSN: 0017-3495 DOI: 10.3989/gya.021109 Oil fatty acid composition of eighteen Mediterranean olive varieties cultivated under the arid conditions of Boughrara (southern Tunisia) By Wissem Zarrouk,a Bechir Baccouri,a Wael Taamalli,a Ahmed Trigui,b Douja Daouda and Mokhtar Zarrouka* a Laboratoire Caractérisation et Qualité de l’Huile d’Olive, Centre de Biotechnologie de Borj Cedria, BP 901, 2050 Hammam-Lif, Tunisia. b Institut de l’Olivier, BP 263, 3018 Sfax, Tunisia. (*Corresponding author: e-mail: [email protected]) RESUMEN three main groups. The first group included a subgroup which is composed of seven olive varieties (Cornezuelo, Ver- Composición de ácidos grasos de dieciocho varieda- dial de Vélez-Málaga, Leccino, Coratina, Koroneiki, Lechín des de aceituna mediterráneas cultivadas en las condi- de Granada and Changlot Real) characterized by high oil ciones áridas de Boughrara (zona meridional de Túnez). yield with high oleic, low palmitic and linoleic acid contents. The fatty acid compositions of the oils from these varieties En este estudio, dieciocho variedades de aceituna pro- comply with international standards and show more benefi- cedentes de España, de Francia, de Italia, de Grecia y de Ar- cial characteristics than the oil obtained from Chemlali: the gelia, cultivadas en la estación experimental del olivo de most abundant olive cultivar in Tunisia. Finally, the main fatty Boughrara (región árida de Túnez), fueron evaluadas para el acids (palmitic (C16:0), oleic (C18:1) and linoleic (C18:2)) of rendimiento en aceite y la composición de ácidos grasos.