TEMPERATURE in the WORKPLACE Anm2 Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture 4: 09.16.05 Temperature, Heat, and Entropy
3.012 Fundamentals of Materials Science Fall 2005 Lecture 4: 09.16.05 Temperature, heat, and entropy Today: LAST TIME .........................................................................................................................................................................................2� State functions ..............................................................................................................................................................................2� Path dependent variables: heat and work..................................................................................................................................2� DEFINING TEMPERATURE ...................................................................................................................................................................4� The zeroth law of thermodynamics .............................................................................................................................................4� The absolute temperature scale ..................................................................................................................................................5� CONSEQUENCES OF THE RELATION BETWEEN TEMPERATURE, HEAT, AND ENTROPY: HEAT CAPACITY .......................................6� The difference between heat and temperature ...........................................................................................................................6� Defining heat capacity.................................................................................................................................................................6� -

The Relative Contributions of Temperature and Moisture to Heat Stress
1 The Relative Contributions of Temperature and Moisture to Heat Stress 2 Changes Under Warming ∗ 3 Nicholas J. Lutsko 4 Scripps Institution of Oceanography, University of California at San Diego, La Jolla, CA, USA ∗ 5 Corresponding author address: Nicholas Lutsko, [email protected] 6 E-mail: [email protected] Generated using v4.3.2 of the AMS LATEX template 1 ABSTRACT 7 Increases in the severity of heat stress extremes are potentially one of the 8 most impactful consequences of climate change, affecting human comfort, 9 productivity, health and mortality in many places on Earth. Heat stress results 10 from a combination of elevated temperature and humidity, but the relative con- 11 tributions each of these makes to heat stress changes have yet to be quantified. 12 Here, conditions on the baseline specific humidity are derived for when spe- 13 cific humidity changes will dominate heat stress changes (as measured using 14 the equivalent potential temperature, qE), and for when temperature changes 15 will dominate. Separate conditions are derived over ocean and over land, in 16 addition to a condition for when relative humidity changes dominate over the 17 temperature response at fixed relative humidity. These conditions are used to 18 interpret the qE responses in transient warming simulations with an ensemble 19 of models participating in the Sixth Climate Model Intercomparison Project. 20 The regional pattern of qE changes is shown to be largely determined by the 21 pattern of specific humidity changes, with the pattern of temperature changes 22 playing a secondary role. This holds whether considering changes in mean 23 summertime qE or in extreme (98th percentile) qE events. -
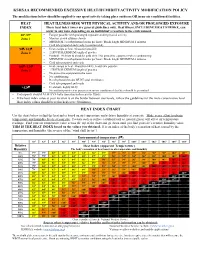
Kshsaa Recommended Excessive Heat/Humidityactivity Modification Policy Heat Index Chart
KSHSAA RECOMMENDED EXCESSIVE HEAT/HUMIDITYACTIVITY MODIFICATION POLICY The modifications below should be applied to any sport/activity taking place outdoors OR in un-air conditioned facilities. HEAT HEAT ILLNESS RISK WITH PHYSICAL ACTIVITY AND/OR PROLONGED EXPOSURE INDEX These heat index zones are general guidelines only. Heat illness, INCLUDING HEAT STROKE, can occur in any zone depending on an individual’s reaction to the environment. 80°-89° – Fatigue possible with prolonged exposure and/or physical activity Zone 1 – Monitor at-risk athletes closely – MINIMUM 3 rest/hydration breaks per hour / Break length MINIMUM 4 minutes – Cold tubs prepared and ready (recommended) 90- 103 – Heat cramps or heat exhaustion possible Zone 2 – 2 HOUR MAXIMUM length of practice – Football: Helmets & shoulder pads only / No protective equipment when conditioning – MINIMUM 4 rest/hydration breaks per hour / Break length MINIMUM 4 minutes – Cold tubs prepared and ready 103- 124 – Heat cramps or heat exhaustion likely, heatstroke possible Zone 3 – 1 HOUR MAXIMUM length of practice – No protective equipment to be worn – No conditioning – Rest/hydration breaks MUST total 20 minutes – Cold tubs prepared and ready >124 – Heatstroke highly likely – No outdoor practices or practices in un-air conditioned facilities should be permitted – Participants should ALWAYS have unrestricted access to fluids. – If the heat index value at your location is on the border between two levels, follow the guidelines for the more conservative level. – Heat index values should be rechecked every 30 minutes. HEAT INDEX CHART Use the chart below to find the heat index based on air temperature and relative humidity at your site. -

Work and Energy Summary Sheet Chapter 6
Work and Energy Summary Sheet Chapter 6 Work: work is done when a force is applied to a mass through a displacement or W=Fd. The force and the displacement must be parallel to one another in order for work to be done. F (N) W =(Fcosθ)d F If the force is not parallel to The area of a force vs. the displacement, then the displacement graph + W component of the force that represents the work θ d (m) is parallel must be found. done by the varying - W d force. Signs and Units for Work Work is a scalar but it can be positive or negative. Units of Work F d W = + (Ex: pitcher throwing ball) 1 N•m = 1 J (Joule) F d W = - (Ex. catcher catching ball) Note: N = kg m/s2 • Work – Energy Principle Hooke’s Law x The work done on an object is equal to its change F = kx in kinetic energy. F F is the applied force. 2 2 x W = ΔEk = ½ mvf – ½ mvi x is the change in length. k is the spring constant. F Energy Defined Units Energy is the ability to do work. Same as work: 1 N•m = 1 J (Joule) Kinetic Energy Potential Energy Potential energy is stored energy due to a system’s shape, position, or Kinetic energy is the energy of state. motion. If a mass has velocity, Gravitational PE Elastic (Spring) PE then it has KE 2 Mass with height Stretch/compress elastic material Ek = ½ mv 2 EG = mgh EE = ½ kx To measure the change in KE Change in E use: G Change in ES 2 2 2 2 ΔEk = ½ mvf – ½ mvi ΔEG = mghf – mghi ΔEE = ½ kxf – ½ kxi Conservation of Energy “The total energy is neither increased nor decreased in any process. -

Wet Bulb Globe Temperature Faqs
3/30/17 3rd Annual Collaborative Solutions for Safety in Sport National Meeting Wet Bulb Globe Temperature FAQs Environmental Monitoring Indices • Wet bulb globe temperature (WBGT) • Air temperature • Relative humidity • Sling psychrometer • Heat index • OSHA chart 1 3/30/17 How are they different? Wet Bulb Globe Temperature • Invented in 1950s for the US Army and Marine Corps • Wet Bulb Temperature (Tw) • Humidity, (Wind) • Globe Temperature (Tg) • Solar radiation, (Wind) • Dry Bulb Temperature (Td) • Air temperature WBGT= 0.7Tw + 0.2Tg + 0.1Td Budd GM. Wet-bulb globe temperature (WBGT)--its history and its limitations. J Sci Med Sport Sports Med Aust. 2008;11(1):20-32. How are they different? Sling Psychrometer • Two thermometers mounted together in the same device. • Calculates the difference between: • Ambient temperature • Wet-bulb thermometer $50- $100/unit • Measures relative humidity • Allows clinician to then derive heat index 2 3/30/17 How are they different? Heat Index • Heat Index is how hot it feels when relative humidity is factored into the ambient temperature. • Heat Index is created based on shady, light-wind conditions. • Not full sunshine • Not strong-wind • Number may NOT be reliable under extreme heat conditions How are they different? Heat Index • Assumptions of Heat Index • Shaded Football helmet • (full sun can increase Heat Index by 15oF) • 5’7”, 147 lbs • Long pants and short sleeve shirtFootball uniform • Walking at 3 mph High physical demand 3 3/30/17 Why WBGT? • WBGT is a more comprehensive representation of environmental conditions • Solar radiation & wind speed are factored into the equation • Devised to account for physical activity Regional Specificity • Regional specific guideline by Grundstein et al. -

Heat Index Climatology for the North-Central United States
HEAT INDEX CLIMATOLOGY FOR THE NORTH-CENTRAL UNITED STATES Todd Rieck National Weather Service La Crosse, Wisconsin 1. Introduction middle Mississippi River Valleys, and the western Great Lakes. Also, the physiological Heat is an underrated danger, with an response to heat will be briefly investigated, average of 175 Americans losing their lives including a review of how heat acclimatization annually from heat-related causes. According to affects the human body’s biology. This the Centers for Disease Control and Prevention, protective biological response is an important from 1979-2003 excessive heat exposure consideration when evaluating the impact of the caused 8,015 deaths in the United States. heat on those that are, or are not, acclimatized During this period, more people died from to the heat. extreme heat than from hurricanes, lightning, tornadoes, and floods combined. In this study, 95°F will be used as the start for the climatological analysis as prolonged Heat kills by taxing the human body beyond exposure to heat this warm increases the risk of its ability to cool itself. Cooling is primarily sunstroke, heat cramps, and heat exhaustion accomplished by the evaporation of perspiration. (Table 1) . How efficiently this process functions is directly related to the amount of water vapor in the air. 2. Data High moisture content reduces the evaporative cooling rate of perspiration, making it difficult for All available weather observations from the the body to maintain a steady and safe internal National Climatic Data Center were used from temperature. One way to measure the 192 locations (Fig. 1), extending from Utah to combined effect of temperature and moisture on Michigan, and from the Canadian-U.S. -

Lecture 8: Maximum and Minimum Work, Thermodynamic Inequalities
Lecture 8: Maximum and Minimum Work, Thermodynamic Inequalities Chapter II. Thermodynamic Quantities A.G. Petukhov, PHYS 743 October 4, 2017 Chapter II. Thermodynamic Quantities Lecture 8: Maximum and Minimum Work, ThermodynamicA.G. Petukhov,October Inequalities PHYS 4, 743 2017 1 / 12 Maximum Work If a thermally isolated system is in non-equilibrium state it may do work on some external bodies while equilibrium is being established. The total work done depends on the way leading to the equilibrium. Therefore the final state will also be different. In any event, since system is thermally isolated the work done by the system: jAj = E0 − E(S); where E0 is the initial energy and E(S) is final (equilibrium) one. Le us consider the case when Vinit = Vfinal but can change during the process. @ jAj @E = − = −Tfinal < 0 @S @S V The entropy cannot decrease. Then it means that the greater is the change of the entropy the smaller is work done by the system The maximum work done by the system corresponds to the reversible process when ∆S = Sfinal − Sinitial = 0 Chapter II. Thermodynamic Quantities Lecture 8: Maximum and Minimum Work, ThermodynamicA.G. Petukhov,October Inequalities PHYS 4, 743 2017 2 / 12 Clausius Theorem dS 0 R > dS < 0 R S S TA > TA T > T B B A δQA > 0 B Q 0 δ B < The system following a closed path A: System receives heat from a hot reservoir. Temperature of the thermostat is slightly larger then the system temperature B: System dumps heat to a cold reservoir. Temperature of the system is slightly larger then that of the thermostat Chapter II. -

Entropy: Is It What We Think It Is and How Should We Teach It?
Entropy: is it what we think it is and how should we teach it? David Sands Dept. Physics and Mathematics University of Hull UK Institute of Physics, December 2012 We owe our current view of entropy to Gibbs: “For the equilibrium of any isolated system it is necessary and sufficient that in all possible variations of the system that do not alter its energy, the variation of its entropy shall vanish or be negative.” Equilibrium of Heterogeneous Substances, 1875 And Maxwell: “We must regard the entropy of a body, like its volume, pressure, and temperature, as a distinct physical property of the body depending on its actual state.” Theory of Heat, 1891 Clausius: Was interested in what he called “internal work” – work done in overcoming inter-particle forces; Sought to extend the theory of cyclic processes to cover non-cyclic changes; Actively looked for an equivalent equation to the central result for cyclic processes; dQ 0 T Clausius: In modern thermodynamics the sign is negative, because heat must be extracted from the system to restore the original state if the cycle is irreversible . The positive sign arises because of Clausius’ view of heat; not caloric but still a property of a body The transformation of heat into work was something that occurred within a body – led to the notion of “equivalence value”, Q/T Clausius: Invented the concept “disgregation”, Z, to extend the ideas to irreversible, non-cyclic processes; TdZ dI dW Inserted disgregation into the First Law; dQ dH TdZ 0 Clausius: Changed the sign of dQ;(originally dQ=dH+AdL; dL=dI+dW) dHdQ Derived; dZ 0 T dH Called; dZ the entropy of a body. -

Lecture 6: Entropy
Matthew Schwartz Statistical Mechanics, Spring 2019 Lecture 6: Entropy 1 Introduction In this lecture, we discuss many ways to think about entropy. The most important and most famous property of entropy is that it never decreases Stot > 0 (1) Here, Stot means the change in entropy of a system plus the change in entropy of the surroundings. This is the second law of thermodynamics that we met in the previous lecture. There's a great quote from Sir Arthur Eddington from 1927 summarizing the importance of the second law: If someone points out to you that your pet theory of the universe is in disagreement with Maxwell's equationsthen so much the worse for Maxwell's equations. If it is found to be contradicted by observationwell these experimentalists do bungle things sometimes. But if your theory is found to be against the second law of ther- modynamics I can give you no hope; there is nothing for it but to collapse in deepest humiliation. Another possibly relevant quote, from the introduction to the statistical mechanics book by David Goodstein: Ludwig Boltzmann who spent much of his life studying statistical mechanics, died in 1906, by his own hand. Paul Ehrenfest, carrying on the work, died similarly in 1933. Now it is our turn to study statistical mechanics. There are many ways to dene entropy. All of them are equivalent, although it can be hard to see. In this lecture we will compare and contrast dierent denitions, building up intuition for how to think about entropy in dierent contexts. The original denition of entropy, due to Clausius, was thermodynamic. -

Appendix a Gempak Parameters
GEMPAK Parameters APPENDIX A GEMPAK PARAMETERS This appendix contains a list of the GEMPAK parameters. Algorithms used in computing these parameters are also included. The following constants are used in the computations: KAPPA = Poisson's constant = 2 / 7 G = Gravitational constant = 9.80616 m/sec/sec GAMUSD = Standard atmospheric lapse rate = 6.5 K/km RDGAS = Gas constant for dry air = 287.04 J/K/kg PI = Circumference / diameter = 3.14159265 References for some of the algorithms: Bolton, D., 1980: The computation of equivalent potential temperature., Monthly Weather Review, 108, pp 1046-1053. Miller, R.C., 1972: Notes on Severe Storm Forecasting Procedures of the Air Force Global Weather Central, AWS Tech. Report 200. Wallace, J.M., P.V. Hobbs, 1977: Atmospheric Science, Academic Press, 467 pp. TEMPERATURE PARAMETERS TMPC - Temperature in Celsius TMPF - Temperature in Fahrenheit TMPK - Temperature in Kelvin STHA - Surface potential temperature in Kelvin STHK - Surface potential temperature in Kelvin N-AWIPS 5.6.L User’s Guide A-1 October 2003 GEMPAK Parameters STHC - Surface potential temperature in Celsius STHE - Surface equivalent potential temperature in Kelvin STHS - Surface saturation equivalent pot. temperature in Kelvin THTA - Potential temperature in Kelvin THTK - Potential temperature in Kelvin THTC - Potential temperature in Celsius THTE - Equivalent potential temperature in Kelvin THTS - Saturation equivalent pot. temperature in Kelvin TVRK - Virtual temperature in Kelvin TVRC - Virtual temperature in Celsius TVRF - Virtual -

Work-Energy for a System of Particles and Its Relation to Conservation Of
Conservation of Energy, the Work-Energy Principle, and the Mechanical Energy Balance In your study of engineering and physics, you will run across a number of engineering concepts related to energy. Three of the most common are Conservation of Energy, the Work-Energy Principle, and the Mechanical Engineering Balance. The Conservation of Energy is treated in this course as one of the overarching and fundamental physical laws. The other two concepts are special cases and only apply under limited conditions. The purpose of this note is to review the pedigree of the Work-Energy Principle, to show how the more general Mechanical Energy Bal- ance is developed from Conservation of Energy, and finally to describe the conditions under which the Mechanical Energy Balance is preferred over the Work-Energy Principle. Work-Energy Principle for a Particle Consider a particle of mass m and velocity V moving in a gravitational field of strength g sub- G ject to a surface force Rsurface . Under these conditions, writing Conservation of Linear Momen- tum for the particle gives the following: d mV= R+ mg (1.1) dt ()Gsurface Forming the dot product of Eq. (1.1) with the velocity of the particle and rearranging terms gives the rate form of the Work-Energy Principle for a particle: 2 dV⎛⎞ d d ⎜⎟mmgzRV+=() surfacei G ⇒ () EEWK += GP mech, in (1.2) dt⎝⎠2 dt dt Gravitational mechanical Kinetic potential power into energy energy the system Recall that mechanical power is defined as WRmech, in= surfaceiV G , the dot product of the surface force with the velocity of its point of application. -

The Heat Index "Equation" (Or, More Than You Ever Wanted to Know About Heat Index)
SR 90-23 Technical Attachment 7/1/90 The Heat Index "Equation" (or, More Than You Ever Wanted to Know About Heat Index) Lans P. Rothfusz Scientific Services Division NWS Southern Region Headquarters, Fort Worth, TX Now that summer has spread its oppressive ridge over most of the Southern Region, NWS phones are ringing off their hooks with questions about the Heat Index. Many questions regard the actual equation used in calculating the Heat Index. Some callers are satisfied with the response that it is extremely complicated. Some are satisfied with the nomogram (see Attachment 1). But there are a few who will settle for nothing less than the equation itself. No true equation for the Heat Index exists. Heat Index values are derived from a collection of equations that comprise a model. This Technical Attachment presents an equation that approximates the Heat Index and, thus, should satisfy the latter group of callers. The Heat Index (or apparent temperature) is the result of extensive biometeorological studies. The parameters involved in its calculation are shown below (from Steadman, 1979). Each of these parameters can be described by an equation but they are given assumed magnitudes (in parentheses) in order to simplify the model. # Vapor pressure. Ambient vapor pressure of the atmosphere. (1.6 kPa) # Dimensions of a human. Determines the skin's surface area. (5' 7" tall, 147 pounds) # Effective radiation area of skin. A ratio that depends upon skin surface area. (0.80) # Significant diameter of a human. Based on the body's volume and density. (15.3 cm) # Clothing cover.