Alteration Assemblages in Martian Meteorites 369
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shergotty Basalt, 5 Kg Seen to Fall Introduction the Shergotty Achondrite Fell on August 25, 1865 at 9:00 A.M
V. Shergotty basalt, 5 kg seen to fall Introduction The Shergotty achondrite fell on August 25, 1865 at 9:00 a.m. near a town called Shergahti in Bihar State, India after detonations were heard (Graham et al. 1985). Duke (1968) refers to several stones with fusion crusts, but this has not been confirmed. The main mass is at the Museum of the Geological Survey in Calcutta, India (figure V-1). In 1984, an international consortium was organized by J. C. Laul to study ~30 grams of Shergotty in detail (Laul 1986a, b). Shergottites (Shergotty, Zagami, EETA79001B, QUE94201, Los Angeles) are texturally and mineralogically similar to terrestrial diabases (although all of the plagioclase has been shocked to maskelynite), but quite distinct petrologically and chemically from the rest of the basaltic achondrites (Stolper et al. 1979). Stolper and McSween (1979) and others have noted that Shergotty crystallized under relatively oxidizing conditions. Figure V-1. Photograph of Shergotty meteorite The Shergotty meteorite has been severely shocked and showing fusion crust and borken surfaces. Two saw is considered the “type locality” for maskelynite (dense cuts are visible. Sample is about 25 cm across. Photo plagioclase glass). In fact, it has proven to be very kindly provided by Prof. N. Bhandari, Director, Physical Research Laboratory, Ahmedabad, India. difficult to date the original crystallization event of Figure V-2. Photograph of slab of Shergotty meteorite showing basaltic texture and alignment of pyroxene crystals. Note the inclusion of black glass in the center of this slab. This is figure 1 in Duke (1968). Photo courtesy of U. -

LEW 88516.Pmd
LEW88516 – 13.2 grams Lherzolitic Shergottite Figure 1. Photograph of LEW88516 after first break for processing. Cube is 1 cm for scale. NASA # S91-37060 Introduction meteorite has three distinct textural regions: 1) poikilitic Lewis Cliff sample LEW88516 is petrologically similar crystalline, 2) interstitial crystalline (or non-poikilitic) to ALH77005 (Satterwhite and Mason 1991; Treiman and, 3) glassy to partially crystalline veinlets. Delaney et al. 1994) and Y793605 (Kojima et al. 1997). (1992), Lindstrom et al. (1992) and Harvey and However, it is not paired, because it has a distinctly McSween (1992) have also reported petrological different terrestrial exposure age. Figure 1 shows descriptions of LEW88516. Papike et al. (2009) LEW88516 as a small (2 cm), dark, rounded, meteorite compared LEW88516 with the rest of the shergottites. with coarsely crystalline interior. In the poikilitic regions, mm-sized olivine crystals and LEW88516 was a small nondescript meteorite that was 50 micron-sized chromite crystals are contained within, not recognized as an achondrite until it was broken open and completely surrounded by, pigeonite crystals with Hence, it waited 2 years to be processed in numerical exsolution lamellae of augite. Maskelynite, whitlockite order. According to Satterwhite and Mason (1991), and sulfides are rare in the poikilitic regions. LEW88516 had a “pitted and mostly shiny fusion crust over 80 % of the surface.” Preliminary The interstitial areas have a more typically basaltic examination of the thin section showed about 50 % texture with intergrown euhedral and subhedral olivine, olivine, 35 % pyroxene, 8 % interstitial maskelynite with pigeonite and chromite, with interstitial maskelynite, about 5% brown glass. Grain size is about 2 - 3 mm. -

Design of Low-Altitude Martian Orbits Using Frequency Analysis A
Design of Low-Altitude Martian Orbits using Frequency Analysis A. Noullez, K. Tsiganis To cite this version: A. Noullez, K. Tsiganis. Design of Low-Altitude Martian Orbits using Frequency Analysis. Advances in Space Research, Elsevier, 2021, 67, pp.477-495. 10.1016/j.asr.2020.10.032. hal-03007909 HAL Id: hal-03007909 https://hal.archives-ouvertes.fr/hal-03007909 Submitted on 16 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Design of Low-Altitude Martian Orbits using Frequency Analysis A. Noulleza,∗, K. Tsiganisb aUniversit´eC^oted'Azur, Observatoire de la C^oted'Azur, CNRS, Laboratoire Lagrange, bd. de l'Observatoire, C.S. 34229, 06304 Nice Cedex 4, France bSection of Astrophysics Astronomy & Mechanics, Department of Physics, Aristotle University of Thessaloniki, GR 541 24 Thessaloniki, Greece Abstract Nearly-circular Frozen Orbits (FOs) around axisymmetric bodies | or, quasi-circular Periodic Orbits (POs) around non-axisymmetric bodies | are of primary concern in the design of low-altitude survey missions. Here, we study very low-altitude orbits (down to 50 km) in a high-degree and order model of the Martian gravity field. We apply Prony's Frequency Analysis (FA) to characterize the time variation of their orbital elements by computing accurate quasi-periodic decompositions of the eccentricity and inclination vectors. -

Indiana Geological and Water Survey Meteorites in Indiana
OGICAL L SU EO R G V E A Y N 1 A 8 I 3 D 7 N Meteorites in Indiana I I N Y D IT IA S N R A UNI VE By Nelson R. Shaffer Meteorites — rocks that fall to Earth from outer Stone meteorites are mineralogically the most complex space, have fascinated mankind since the beginning and are the most abundant. They are dominantly of time. These scientifically valuable objects help made of silicates. Two main types—chondrites and geologists understand the origins of planets and the achondrites—are recognized. processes that shape the Earth. Meteorites are rare and exhibit special features that differentiate them from Chondrites, the most common (84 percent of falls), Earth rocks. Among the characteristics that identify contain small (less than 1/8 inch) structured spheres meteorites are a high specific gravity (especially true called chondrules. Chondrules are found only in for irons), a dark color, and a dark glassy or dull crust meteorites and contain some of the oldest material if fresh or a rind of iron oxide (rust) if weathered. known to Man. Their origin is still uncertain, despite Most meteorites attract a magnet, although some only many theories proposed to explain them. slightly. Many have an aerodynamic shape, and their Achondrites—the second type of stone meteorites— crusts may be marked with flow structures (see photo contain silicates but do not contain chondrules. They of Lafayette meteorite) or shallow depressions called resemble basalt from the Earth and represent about 8 “thumbprints” (see the South Bend meteorite). percent of falls. -

Planting and Germination of Sweet Potato, Yam and Radish Plants in the Mars Desert Research Station
Copyright © 2015 by Olenka Jibaja Valderrama. Published by The Mars Society with permission. Planting and Germination of Sweet Potato, Yam and Radish Plants in the Mars Desert Research Station Olenka Jibaja Valderrama Universidad Católica Santo Toribio de Mogrovejo – Chiclayo, Perú [email protected] Abstract: As part of the future exploration of the universe, manned missions to other planets or satellites near Earth will be needed. One of the main objectives of these missions will be to reach Mars because it is at a relatively close distance to our planet and because both planets have some common characteristics. Since the economic investment of these explorations is quite high, the duration of them must be significant; that’s why the missions should aim to be self-sufficient. The search for food sources for crew members is a key factor to be investigated and even more attention should be on food whose production is possible and sustainable in the environment where the mission arises. This work was involved with the planting and germination of radish, yam and sweet potato plants in the greenhouse of the Mars Desert Research Station (MDRS) and the main objective was to investigate the characteristics of growth and development of these plants in Martian conditions to find out whether its production is possible or not. Orange skin and white skin: two types of sweet potatoes were planted. During rotation, it was discovered that yam grew faster under these conditions than the rest of the tubers, although its development was slower as compared to terrestrial conditions. Radish plants in Mars regolith grew in a similar speed than in the fertile Earth soil, and even faster than in drier soil taken from the desert. -
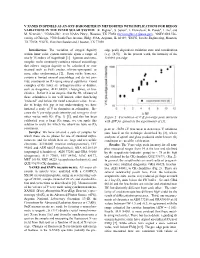
V Xanes in Spinels As an Oxy-Barometer in Meteorites with Implications for Redox Variations in the Inner Solar System
V XANES IN SPINELS AS AN OXY-BAROMETER IN METEORITES WITH IMPLICATIONS FOR REDOX VARIATIONS IN THE INNER SOLAR SYSTEM. K. Righter1, S. Sutton2, L. Danielson3, K. Pando4, L. Le3, and M. Newville2. 1NASA-JSC, 2101 NASA Pkwy., Houston, TX 77058 ([email protected]), 2GSECARS Uni- versity of Chicago, 9700 South Cass Avenue, Bldg. 434A, Argonne, IL 60439; 3ESCG, Jacobs Engineering, Houston, TX 77058; 4ESCG, Hamilton Sundstrand, Houston, TX 77058 Introduction: The variation of oxygen fugacity edge peak) depend on oxidation state and coordination within inner solar system materials spans a range of (e.g., [4,5]). In the present work, the intensity of the nearly 15 orders of magnitiude [1]. Igneous and meta- XANES pre-edge morphic rocks commonly contain a mineral assemblage that allows oxygen fugacity to be calculated or con- strained such as FeTi oxides, olivine-opx-spinel, or some other oxybarometer [2]. Some rocks, however, contain a limited mineral assemblage and do not pro- vide constraints on fO2 using mineral equilibria. Good examples of the latter are orthopyroxenites or dunites, such as diogenites, ALH 84001, chassignites, or bra- chinites. In fact it is no surprise that the fO2 of many of these achondrites is not well known, other than being "reduced" and below the metal saturation value. In or- der to bridge this gap in our understanding, we have initiated a study of V in chromites in achondrite. Be- cause the V pre-edge peak intensity and energy in chro- mites varies with fO2 (Fig. 1) [3], and this has been Figure 1: Correlation of V K pre-edge peak intensity calibrated over a large fO2 range, we can apply this with IW for spinels in the experiments of [3]. -

Cosmic-Ray-Exposure Ages Martian Meteorites Million Years
Introduction to MMC 2012 someone keeps adding pieces as you go along (see my Introduction 2006). Most Martian meteorites are mafic As of the end of 2012, we now know of about 65 basalts. That is, they have an abundance of Fe and different meteorites from Mars with a total weight about Mg, are low in Si and Al and have a texture of 120 kilograms. Some fell as showers (e.g. Nakhla, intergrown minerals typical of terrestrial basalts (the Tissint), and some can be paired by common lithology, tradition has been to call them “shergottites”, after the age and fall location, but it is more instructive to first sample recognized as such). Some often have an consider how they are grouped by “launch pairs”. If abundance of large olivine crystals (so called olivine- you add the terrestrial age to the cosmic exposure age phyric). Others have an abundance of both high- and (CRE) you get the time when the meteorite was low-Ca pyroxene, with poikilitic textures and were launched off of Mars by impact. Since only rather large originally called “lherzolitic shergottites”. But it has impacts would do this, there must be a finite number now been recognized that there is a more fundamental of such impacts – hence grouping of samples. and better way to describe these rocks (Irving 2012). Now that we have such a large number of samples, it Figure 1 shows a crude summary of CRE that have is time to understand what we have and infer what we been determined for Martian meteorites. Note that can about Mars. -

Variability of Mars' North Polar Water Ice Cap I. Analysis of Mariner 9 and Viking Orbiter Imaging Data
Icarus 144, 382–396 (2000) doi:10.1006/icar.1999.6300, available online at http://www.idealibrary.com on Variability of Mars’ North Polar Water Ice Cap I. Analysis of Mariner 9 and Viking Orbiter Imaging Data Deborah S. Bass Instrumentation and Space Research Division, Southwest Research Institute, P.O. Drawer 28510, San Antonio, Texas 78228-0510 E-mail: [email protected] Kenneth E. Herkenhoff U.S. Geological Survey, 2255 North Gemini Drive, Flagstaff, Arizona 86001 and David A. Paige Department of Earth and Space Sciences, University of California, Los Angeles, 405 Hilgard Avenue, Los Angeles, California 90095-1567 Received May 15, 1998; revised November 17, 1999 1. INTRODUCTION Previous studies interpreted differences in ice coverage between Mariner 9 and Viking Orbiter observations of Mars’ north residual Like Earth, Mars has perennial ice caps and an active wa- polar cap as evidence of interannual variability of ice deposition on ter cycle. The Viking Orbiter determined that the surface of the the cap. However,these investigators did not consider the possibility northern residual cap is water ice (Kieffer et al. 1976, Farmer that there could be significant changes in the ice coverage within et al. 1976). At the south residual polar cap, both Mariner 9 the northern residual cap over the course of the summer season. and Viking Orbiter observed carbon dioxide ice throughout the Our more comprehensive analysis of Mariner 9 and Viking Orbiter summer. Many have related observed atmospheric water vapor imaging data shows that the appearance of the residual cap does not abundances to seasonal exchange between reservoirs such as show large-scale variance on an interannual basis. -

Linking the Chassigny Meteorite and the Martian Surface Rock Backstay: Insights Into Igneous Crustal Differentiation Processes on Mars
Meteoritics & Planetary Science 44, Nr 6, 853–869 (2009) Abstract available online at http://meteoritics.org Linking the Chassigny meteorite and the Martian surface rock Backstay: Insights into igneous crustal differentiation processes on Mars Hanna NEKVASIL*, Francis M. MCCUBBIN, Andrea HARRINGTON, Stephen ELARDO, and Donald H. LINDSLEY Department of Geosciences, Stony Brook University, Stony Brook, New York 11794–2100, USA *Corresponding author. E-mail: [email protected] (Received 05 August 2008; revision accepted 18 March 2009) Abstract–In order to use igneous surface lithologies to constrain Martian mantle characteristics, secondary processes that lead to compositional modification of primary mantle melts must be considered. Crystal fractionation of a mantle-derived magma at the base of the crust followed by separation and ascent of residual liquids to the surface is common in continental hotspot regions on Earth. The possibility that this process also takes place on Mars was investigated by experimentally determining whether a surface rock, specifically the hawaiite Backstay analyzed by the MER Spirit could produce a known cumulate lithology with a deep origin (namely the assemblages of the Chassigny meteorite) if trapped at the base of the Martian crust. Both the major cumulus and melt inclusion mineral assemblages of the Chassigny meteorite were produced experimentally by a liquid of Backstay composition within the pressure range 9.3 to 6.8 kbar with bulk water contents between 1.5 and 2.6 wt%. Experiments at 4.3 and 2.8 kbar did not produce the requisite assemblages. This agreement suggests that just as on Earth, Martian mantle-derived melts may rise to the surface or remain trapped at the base of the crust, fractionate, and lose their residual liquids. -

Lafayette - 800 Grams Nakhlite
Lafayette - 800 grams Nakhlite Figure 1. Photograph showing fine ablation features Figure 2. Photograph of bottom surface of Lafayette of fusion crust on Lafayette meteorite. Sample is meteorite. Photograph from Field Museum Natural shaped like a truncated cone. This is a view of the top History, Chicago, number 62918. of the cone. Sample is 4-5 centimeters across. Photo- graph from Field Museum Natural History, Chicago, number 62913. Introduction According to Graham et al. (1985), “a mass of about 800 grams was noticed by Farrington in 1931 in the geological collections in Purdue University in Lafayette Indiana.” It was first described by Nininger (1935) and Mason (1962). Lafayette is very similar to the Nakhla and Governador Valadares meteorites, but apparently distinct from them (Berkley et al. 1980). Lafayette is a single stone with a fusion crust showing Figure 3. Side view of Lafayette. Photograph from well-developed flow features from ablation in the Field Museum Natural History, Chicago, number Earth’s atmosphere (figures 1,2,3). The specimen is 62917. shaped like a rounded cone with a blunt bottom end. It was apparently oriented during entry into the Earth’s that the water released during stepwise heating of atmosphere. Note that the fine ablation features seen Lafayette was enriched in deuterium. The alteration on Lafayette have not been reported on any of the assemblages in Lafayette continue to be an active field Nakhla specimens. of research, because it has been shown that the alteration in Lafayette occurred on Mars. Karlsson et al. (1992) found that Lafayette contained the most extra-terrestrial water of any Martian Lafayette is 1.32 b.y. -

Physical Properties of Martian Meteorites: Porosity and Density Measurements
Meteoritics & Planetary Science 42, Nr 12, 2043–2054 (2007) Abstract available online at http://meteoritics.org Physical properties of Martian meteorites: Porosity and density measurements Ian M. COULSON1, 2*, Martin BEECH3, and Wenshuang NIE3 1Solid Earth Studies Laboratory (SESL), Department of Geology, University of Regina, Regina, Saskatchewan S4S 0A2, Canada 2Institut für Geowissenschaften, Universität Tübingen, 72074 Tübingen, Germany 3Campion College, University of Regina, Regina, Saskatchewan S4S 0A2, Canada *Corresponding author. E-mail: [email protected] (Received 11 September 2006; revision accepted 06 June 2007) Abstract–Martian meteorites are fragments of the Martian crust. These samples represent igneous rocks, much like basalt. As such, many laboratory techniques designed for the study of Earth materials have been applied to these meteorites. Despite numerous studies of Martian meteorites, little data exists on their basic structural characteristics, such as porosity or density, information that is important in interpreting their origin, shock modification, and cosmic ray exposure history. Analysis of these meteorites provides both insight into the various lithologies present as well as the impact history of the planet’s surface. We present new data relating to the physical characteristics of twelve Martian meteorites. Porosity was determined via a combination of scanning electron microscope (SEM) imagery/image analysis and helium pycnometry, coupled with a modified Archimedean method for bulk density measurements. Our results show a range in porosity and density values and that porosity tends to increase toward the edge of the sample. Preliminary interpretation of the data demonstrates good agreement between porosity measured at 100× and 300× magnification for the shergottite group, while others exhibit more variability. -
![Wednesday, March 22, 2017 [W453] MARTIAN METEORITE MADNESS: MIXING on a VARIETY of SCALES 1:30 P.M](https://docslib.b-cdn.net/cover/9633/wednesday-march-22-2017-w453-martian-meteorite-madness-mixing-on-a-variety-of-scales-1-30-p-m-489633.webp)
Wednesday, March 22, 2017 [W453] MARTIAN METEORITE MADNESS: MIXING on a VARIETY of SCALES 1:30 P.M
Lunar and Planetary Science XLVIII (2017) sess453.pdf Wednesday, March 22, 2017 [W453] MARTIAN METEORITE MADNESS: MIXING ON A VARIETY OF SCALES 1:30 p.m. Waterway Ballroom 5 Chairs: Arya Udry Geoffrey Howarth 1:30 p.m. Nielsen S. G. * Magna T. Mezger K. The Vanadium Isotopic Composition of Mars and Evidence for Solar System Heterogeneity During Planetary Accretion [#1225] Vanadium isotope composition of Mars distinct from Earth and chondrites. 1:45 p.m. Tait K. T. * Day J. M. D. Highly Siderophile Element and Os-Sr Isotope Systematics of Shergotittes [#3025] The shergottite meteorites represent geochemically diverse, broadly basaltic, and magmatically-derived rocks from Mars. New samples were processed and analyzed. 2:00 p.m. Armytage R. M. G. * Debaille V. Brandon A. D. Agee C. B. The Neodymium and Hafnium Isotopic Composition of NWA 7034, and Constraints on the Enriched End-Member for Shergottites [#1065] Couple Sm-Nd and Lu-Hf isotopic systematics in NWA 7034 suggest that such a crust is not the enriched end-member for shergottites. 2:15 p.m. Howarth G. H. * Udry A. Nickel in Olivine and Constraining Mantle Reservoirs for Shergottite Meteorites [#1375] Ni enrichment in olivine from enriched versus depleted shergottites provide evidence for constraining mantle reservoirs on Mars. 2:30 p.m. Jean M. M. * Taylor L. A. Exploring Martian Mantle Heterogeneity: Multiple SNC Reservoirs Revealed [#1666] The objective of the present study is to assess how many mixing components can be recognized, and address ongoing debates within the martian isotope community. 2:45 p.m. Udry A. * Day J.