An Analysis of Water Quality Parameters of Vennaimalai in Karur District, Tamilnadu, India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
List of Validated Suppliers-LLIN
List of Validated Suppliers-LLIN Approval Product Reference Approval Material Description Reference Review date Supplier ref No. Supplier Supplier's full address Supplier's Approved Manufacturing site address number date number Rectangular polyester mosquito net/bednet, 75-100 denier, treated with P.O. Box 50606 UAE - The United Arab S0000981 WHOPES recommended insecticide, colour: white/blue/green, dimensions 42105536 01-Jan-18 30-Dec-17 1900019435 Tana Netting FZ-LLC 4-KM, Kahna Kacha Road, Off Ferozepur Road, Lahore, Pakistan Emirates 180x160x150cm (length x width x height) +/-5% Rectangular polyester mosquito net/bednet, 75-100 denier, treated with P.O. Box 50606 UAE - The United Arab S0000982 WHOPES; dimensions190x180x150cm (length x width x height) 42105536 01-Jan-18 30-Dec-17 1900019435 Tana Netting FZ-LLC 4-KM, Kahna Kacha Road, Off Ferozepur Road, Lahore, Pakistan Emirates Conical polyester mosquito net/bednet, 75-100 denier, treated with P.O. Box 50606 UAE - The United Arab S0000983 WHOPES recommended insecticide, colour: white/blue/green, dimensions 42105536 01-Jan-18 30-Dec-17 1900019435 Tana Netting FZ-LLC 4-KM, Kahna Kacha Road, Off Ferozepur Road, Lahore, Pakistan Emirates 1050x220cm (circumference x height) +/-5% Rectangular polyester mosquito net/bednet, 75-100 denier, treated with P.O. Box 50606 UAE - The United Arab S0000984 WHOPES recommended insecticide, colour: white/blue/green, available in 42105536 01-Jan-18 30-Dec-17 1900019435 Tana Netting FZ-LLC 4-KM, Kahna Kacha Road, Off Ferozepur Road, Lahore, Pakistan Emirates alternative dimensions Vestergaard Group SA, 10-10 Textile Joint Stock Co, 253 Minh Khai-Hai Ba Rectangular polyester mosquito net/bednet, 75-100 denier, treated with Place Saint Francois 1, 1003 Lausanne, Trung, Hanoi, Vietnam ,and S0000981 WHOPES recommended insecticide, colour: white/blue/green, dimensions 42105927 01-Jan-18 30-Dec-17 190002051 Vestergaard S.A. -

Provision of Infrastructural Facilities at The
GOVERNMENT OF TAMIL NADU ABSTRACT Tourism – Provision of Infrastructural facilities at the Pilgrim Centres in Karur District – Administrative approval for Rs.9.00 lakhs (Rupees 6.75 lakhs from District Administration and Rs.2.25 lakhs from State funds) – Sanction of Rs.2.25 lakhs – Orders – Issued. INFORMATION AND TOURISM (T3) DEPARTMENT G.O. (Ms) No.42 Date: 25.02.2004. Read: From the Commissioner of Tourism, Chennai – 2. D.O. Letter No.10733/P&D II/2003, dated 6.01.2004. ------ ORDER: In order to have more tourists to Tamil Nadu, the Tourist Centres are to be developed with infrastructure facilities of high standard and the facilities to be developed should be a long term utility for tourism. The State Government has taken steps to strengthen the existing infrastructure at the tourist destinations and to identify the areas of Tourist importance and to develop them with adequate infrastructure for the benefit of Tourists. The Commissioner of Tourism has therefore forwarded the proposal of the District Collector, Karur for provision of infrastructure facilities at a cost of Rs.9.00 lakhs at the following pilgrim centres in Karur District :- a. Vennaimalai Arulmigu Balasubramaniya Swamy Temple, Karur Panchayat – Rs.3.00 lakhs. b. Thirumukkudalur Arulmigu Agastheeswarar Temple, Somur Panchayat.- Rs.3.00 lakhs. c. T.Ayyarmalai Arulmigu Rathinakreeswarar Temple, Sathiyamangalam Panchayat – Rs. 3.00 lakhs. 2. The Commissioner of Tourism has further stated that out of the estimated cost of Rs.9.00 lakhs, the District Collector has given undertaking to meet Rs.6.75 lakhs from the District Adminstration fund and has requested the Government to sanction of Rs.2.25 lakhs from State fund. -

DEPARTMENT of AGRICULTURE KARUR DISTRICT the Government
DEPARTMENT OF AGRICULTURE KARUR DISTRICT The Government have resolved to initiate Second Green Revolution Movement in Tamil Nadu to improve the economic status of the farmers by adopting frontier technologies in a larger extent for various crops with full involvement of farmers and extension staff, backed by research. The major strategies to spur SECOND GREEN REVOLUTION are- • Crop specific strategies to bridge the yield gap, improving agriculture marketing infrastructure and promoting farmer’s participation in agri-business ventures. • Increasing the cultivable area and diversifying the cultivation in favour of high value horticulture and commercial crops while ensuring the food and nutritional security. • Soil health improvement. • Promoting Hi-tech agriculture, Precision Farming and Micro irrigation for efficient use of irrigation water. • Strengthening research and extension services to all farm families in Tamil Nadu-Farm level interventions for “end-to-end” involvement of Extension staff with individual farmer. Soil Type The major soil series in Karur district are Irugur and Thulukkanur .The Soils are generally sandy loam and clay type.The Irugur soil is red to dark red,fine loamy,non calcareous,well drained,slightly acidic to neutral soils. TheTulukkanur soil is reddish brown to dark grayish brown,moderately deep to very deep,fine loamy,calcareous,well drained soils. The other soil series are Palladam,Palathurai,palaviduthi, Vannapatti and Mixed alluvium. Rainfall The average rainfall of the Karur district is 652.2 mm. The major rainfall occurs during north-east monsoon. Irrigation Karur and Manmangalam taluk is being covered by Amaravathy and Cauvery irrigation channel. Aravakurichi Taluk is being covered by Amaravathy and Lower Bhavani Project Channel. -

Karur District
DISTRICT STATISTICAL HAND BOOK KARUR DISTRICT 2016 1 PREFACE The current issue viz “Statistical Hand Book of Karur District 2016 provides latest Statistical data on various Socio‐Economic aspects of Karur District. It contains Key Statistical Information relating to different sectors of Karur District. The Statistical Tables presented in this publication really serve as a useful appropriation for those who are interested in better understanding of the district. Data incorporated in this hand book were collected from various of State and Central Government departments, Public Sector Undertakings and Various Private Institutions. This Hand Book will surely be helpful to have a glance of the District and to serve as a ready reference to Administrators, Planners, Economists, Academicians, Research Scholars, General Public and Social organizations with its wealth of information relating to Demography, Agriculture, Animal Husbandry, Co‐ Operation, Education, Industries etc. I express my sincere gratitude to the District Collector who extended her support for the timely publication of this Hand Book. The co‐operation extended by the various Departments in furnishing the data is gratefully acknowledged with thanks. I also thanks extend to the staff of the District Statistical Unit who toiled hard in preparing this Hand Book. I solicit positive suggestions and comments from the users of this issue for its further improvement . Station : Karur Deputy Director of Statistics Date : Karur 2 SALIENT FEATURES Historical Background about the District : KARUR district was carved out from the erestwhile Thiruchirapalli district. It occupies a very significant place in the history and culture of the Tamils, with a record of achievements spanning over a period of 2000 years. -

BEFORE the NATIONAL GREEN TRIBUNAL SOUTHERN ZONE, CHENNAI. APPLICATION NO. 273 of 2014 (SZ) in the MATTER OF: M/S. Tamil Nadu H
BEFORE THE NATIONAL GREEN TRIBUNAL SOUTHERN ZONE, CHENNAI. APPLICATION NO. 273 OF 2014 (SZ) IN THE MATTER OF: M/s. Tamil Nadu HDPE Knitted Fabrics Manufacturers Association Rep. by its President C. Sureshkumar 19 & 20, Athur SIDCO Industrial Estate Vennaimalai Post, Karur – 639 006. .. Applicant Versus 1. The Secretary, Central Insecticides Board and Registration Committee, Ministry of Agriculture, NH 4, CGO Complex, Faridabad – 121 001. 2. The Deputy Director, Plant Protection Department of Agriculture, Ezhilagam, Chepauk, Chennai – 600 005. 3. The Principal Secretary to Government- cum - Agricultural Production Commissioner Fort St. George, Chennai – 600 009. 4. The Member Secretary Tamil Nadu Pollution Control Board 76, Mount Salai, Guindy, Chennai – 600 032 5. M/s. Shobikaa Impex Private Ltd., Rep. by its Director, Mr. M. Sivasamy Reg. Office: 34, Sannathi Street, Vennaimalai Post, Karur – 639 006. 6. M/s. V.K.A Polymers Private Ltd., Rep. by its Director, Mr. A. Karuppannan Reg. Office: 3-A, Covai Road, Sengunthapuram, Karur – 639 002. .. Respondents (The 5th and 6th Respondents have been impleaded vide orders of the Tribunal dated 05.03.2015 in M.A. Nos. 1 and 2 of 2015(SZ). Page 1 of 16 Counsel appearing for the Applicant: M/s C. Prasanna Venkatesh, Jayanthi Venkatesh and S. Purushothaman Counsel appearing for the Respondents: Smt. C. Sangamithrai for Respondent No.1; M/s. M.K.Subramaniam for Respondent Nos. 2 and 3; Smt. H. Yasmeen Ali for Respondent No.4; M/s. Rajmakesh for Respondent No.5 and M/s. Guruprasad for Respondent No.6. ORDER PRESENT: 1. Hon’ble Justice M. -

District Disaster Management Plan - 2019
DISTRICT DISASTER MANAGEMENT PLAN - 2019 Thiru. T.ANBALAGAN, I.A.S Chairman & District Collector District Disaster Management Authority, Karur District. DISTRICT DISASTER MANAGEMENT PLAN - 2019 Page Chapter Title No. I Profile of the District 1 II District Disaster Management Plan (DDMP) 15 Hazard, Vulnerability, Capacity and Risk III 24 Assessment IV Institutional Mechanism 64 V Preventive and Mitigation Measures 72 VI Preparedness Measures 81 VII Response, Relief and Recovery Measures 99 Coordination Mechanism for Implementation of VIII 106 DDMP Standard Operating Procedures (sops) and Check IX 115 List x Sendai Framework Project - Action Taken Report 126 ANNEXURES 1 First Responders, NGO, Nodal Officers, Zonal Team 132 2 MAPS 150 3 IMPORTANT G.Os 163 4 Contact Numbers 166 Abbreviations BMPTC Building Material and Technology Promotion Council CBDRM Community Based Disaster Risk Management CBR Chemical-Biological-Radiological CF Critical Facilities CI Critical Infrastructure CoR Commissioner of Relief CWC Central Water Commission DC District Collector DDMA District Disaster Management Authority DDMP District Disaster Management Plan DEOC District Emergency Operations Centre DM Disaster Management EOC Emergency Operations Centre F&CS Food and Civil Supplies GDP Gross Domestic Product IAP Incident Action Plan IMD Indian meteorological Department NCC National Cadet Corps NDMA National Disaster Management Authority NDVI Normalized Difference Vegetation Index NGO Non-Governmental Organizations NH National Highways NRSC National Remote Sensing Centre PPP Public Private Partnerships RO Response Officer SDMA State Disaster Management Authority SEOC State Emergency Operations Centre SOP Standard Operating Procedures TNPL Tamil Nadu Newsprint and Paper Limited UNISDR United Nations Office for Disaster Risk Reduction Karur District Disaster Management Plan- 2019 CHAPTER-I PROFILE OF THE DISTRICT 1.1 Geographical Location Karur district is the 27th largest district by area in the State of Tamil Nadu. -
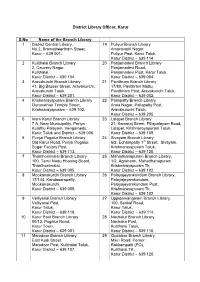
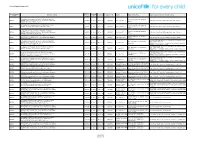
District Library Officer, Karur S.No Name of the Branch Library 1
District Library Officer, Karur S.No Name of the Branch Library 1 District Central Library, 19 Puliyur Branch Library No.2, Bramatheertham Street, Amaravathi Nagar, Karur – 639 001. Puliyur Post, Karur Taluk, Karur District – 639 114. 2 Kulithalai Branch Library 20 Panjamadevi Branch Library 2, Cauvery Nagar, Panjamadevi Road, Kulithalai, Panjamadevi Post, Karur Taluk, Karur District – 630 104. Karur District – 639 004. 3 Aravakurchi Branch Library 21 Pavithram Branch Library 41, Big Bazeer Street, Aravakurchi, 17/80, Pavithram Mettu, Aravakurchi Taluk, Pavithiram Post, Aravakurichi Taluk, Karur District – 639 201. Karur District – 639 002. 4 Krishnarayapuram Branch Library 22 Pallapatty Branch Library Gurunathan Temple Street, Anna Nagar, Pallapatty Post, Krishnarayapuram – 639 102. Aravakuruchi Taluk, Karur District – 639 205. 5 Inam Karur Branch Library 23 Lalapet Branch Library 7 A, Near Municipality, Periya 21, Kamaraj Street, Pillapalayam Road, Kulathu Palayam, Vengamedu, Lalapet, Krishnarayapuram Taluk, Karur Taluk and District – 639 006. Karur District – 639 105. 6 Punjai Pugalur Branch Library 24 Sivayam Branch Library Old Karur Road, Punjai Pugalur, 6/2, Echampatty 1st Street, Sivayam, Sugar Factory Post, Krishnarayapuram Taluk, Karur District – 639 113. Karur District – 639 120. 7 Thanthonimalai Branch Library 25 Mahadhanapuram Branch Library, 100, Tamil Nadu Housing Board, 1/2, Agraharm, Mahadhanapuram, Thanthonimalai, Krishnarayapuram Tk, Karur District – 639 005. Karur District – 639 102. 8 Mookanakurchi Branch Library 26 Palayajeyankondam Branch Library, 17/144, Kandasarapatty, Palayajeyamkondam, Mookanakurchi, Palayajeyamkondam Post, Karur District – 639 005. Krishnarayapuram Tk, Karur District – 639 102. 9 Velliyanai Branch Library 27 Uppidamangalam Branch Library, Velliyanai Post, 100, Senkal Road, Karur Taluk, Karur Taluk, Karur District – 639 118. Karur District - 639 114. -
Are You Suprised ?
1 HINDU RELIGIOUS & CHARITABLE ENDOWMENT DEPARTMENT Arul Migu Balasubramaniya Swamy thirukkoil at Vennaimalai, Karur taluk, in Karur District. TENDER SCHEDULE Name of work : Construction of Ground and First Floor Marriage Hall Building With Dinning Hall after Demolished the Existing Building Structure to Arul Migu Balasubramaniya Swamy thirukkoil at Vennaimalai, Karur taluk in Thiruchirap palli District. Date of Tender : 04.07.2019 E. M. D. : Rs. 49065/- Estimate Amount : Rs 78,13,000/- CONTRACTOR no of corrections: Executive Officer, no of over writings: A/M Balasubramaniya swamy thirukkoil, Vennaimalai, Karur Taluk and District., 2 ANEXURE(TO BE FILLED BY THE CONTRACTOR) 1 Name of Tenderer : 2 Name of Work : Construction of Ground and First Floor Marriage Hall Building With Dinning Hall after Demolished the Existing Building Structure to A/M Balasubramaniya Swamy thirukkoil at Vennaimalai, Karur taluk, in Trichy District. 3 Date of Tender : 04.07.2019 a) In Person : b) By registered post : 4 Total value of tender : 5 Details about E.M.D. enclosed for : this tender and its validity. 6 Registered class of the tenderer : with monetary limit and Department in which registered (Certified copy of the registration should be attached) 7 Recent works executed details : about name and place of work, Agt. No and value of works etc., should be given. 8 Works under execution details about name and place of work Agt. No. & value of works etc., should be furnished. 9 Command of labour in brief. 10 Turn over of previous years : (Particulars for a period of three consecutive years are to be furnished) 11 Whether current Income Tax : clearance certificate is enclosed? If not, when it will be produced? 12 Whether current sales tax clearance certificate is enclosed? If Not, when it will be produced? 13 Sales tax Registration No. -
![KARUR DISTRICT [REVISED FEE STRUCTURE for APPEALED SCHOOLS] S.No C.Code School Name LKG UKG I II III IV V VI VII VIII IX X XI XI](https://docslib.b-cdn.net/cover/0803/karur-district-revised-fee-structure-for-appealed-schools-s-no-c-code-school-name-lkg-ukg-i-ii-iii-iv-v-vi-vii-viii-ix-x-xi-xi-7750803.webp)
KARUR DISTRICT [REVISED FEE STRUCTURE for APPEALED SCHOOLS] S.No C.Code School Name LKG UKG I II III IV V VI VII VIII IX X XI XI
18 - KARUR DISTRICT [REVISED FEE STRUCTURE FOR APPEALED SCHOOLS] S.No C.Code School Name LKG UKG I II III IV V VI VII VIII IX X XI XI BHARATHI NURSERY & PRIMARY SCHOOL, 1 18001 NF NF NF NF NF NF NF NF NF NF NF NF NF NF MINNAMPALLI, PANCHAMADEVI, KARUR NATIONAL NURSERY AND PRIMARY SCHOOL, 2 18003 THALAVAPALAYAM MAIN ROAD, NF NF NF NF NF NF NF NF NF NF NF NF NF NF THALAVAPALAYAM, KARUR (DT) BHARATHI NURSERY & PRIMARY SCHOOL, BHARATHI NAGAR, 3 18004 2800 2800 3300 3300 3300 3300 3300 - - - - - - - NEAR MARIYAMMAN KOVIL,, KARUR,DINDIGUL MAIN ROAD, VELLIYANAI MACMILLAN NURSURY & PRIMARY SCHOOL, NORTH GANDHIGRAMAM, 4 18005 5350 5350 5250 5250 5250 5250 5250 - - - - - - - 474/4 II CROSS PERIYAR NAGAR, PASUPATHIPALAYAM, KARUR 639 004 BRINDAVAN NURSERY & PRIMARY SCHOOL, 5 18008 GANDHIGRAMAM SOUTH, 4250 4250 4050 4050 4050 4050 4050 - - - - - - - KARUR-639004, SARASWATHI VIDYALAYA NURSERY & PRIMARY SCHOOL, 6 18009 KSV NAGAR, THULASIKODUMBU, NF NF NF NF NF NF NF NF NF NF NF NF NF NF JEGADABI KARUR INDIAN NATIONAL NURSERY & PRIMARY SCHOOL, 7 18012 P.VELLALAPATTI ROAD, NF NF NF NF NF NF NF NF NF NF NF NF NF NF PULIYUR, KARUR CHERAN NURSERY AND PRIMARY SCHOOL, 8 18014 3850 3850 4700 4700 4700 4700 4700 - - - - - - - PUNNAMCHATHIRAM, KARUR, ANNAI INDIRA MEMORIRAL HR SEC SCHOOL, 9 18018 LALAPET, KRISHNARAYAPURAM TK, 1900 1900 2300 2300 2300 2300 2300 - - - - - - - KARUR-5 Note:- NF - There is no Recognition, Hence No Fee is Fixed. 18 - KARUR DISTRICT [REVISED FEE STRUCTURE FOR APPEALED SCHOOLS] S.No C.Code School Name LKG UKG I II III IV V VI VII VIII -

District Statistical Hand Book Karur
DISTRICT STATISTICAL HAND BOOK KARUR 2015 Deputy Director of Statistics, Karur. 1 DISTRICT STATISTICAL HAND BOOK KARUR 2015 Deputy Director of Statistics, Karur. 2 Bus Body Building in Karur 3 Weaving Preparatory works by a man 4 Tamil Nadu NewsPrint & Paper Ltd., Kagithapuram. 5 Karur District Blocks 6 PREFACE The current issue of “Statistical Hand Book of Karur District 2016 – 17 provides latest Statistical data on various Socio-Economic aspects of Karur District. It contains Key Statistical Information relating to different sectors of Karur District. The Statistical Tables presented in this publication really serve as a useful appropriation for those who are interested in better understanding of the district. Data incorporated in this hand book were collected from various of State and Central Government departments, Public Sector Undertakings and Various Private Institutions. This Hand Book will surely be helpful to have a glance of the District and to serve as a ready reference to Administrators, Planners, Economists, Academicians, Research Scholars, General Public and Social organizations with its wealth of information relating to Demography, Agriculture, Animal Husbandry, Co- Operation, Education, Industries etc. I express my sincere gratitude to the District Collector who extended her support for the timely publication of this Hand Book. The co-operation extended by the various Departments in furnishing the data is gratefully acknowledged with thanks. I also thanks extend to the staff of the District Statistical Unit who toiled hard in preparing this Hand Book. I solicit positive suggestions and comments from the users of this issue for its further improvement . Station : Karur Deputy Director of Statistics Date : 25.10.2017 Karur 7 SALIENT FEATURES Historical Background about the District : KARUR district was carved out from the erstwhile Thiruchirapalli district. -
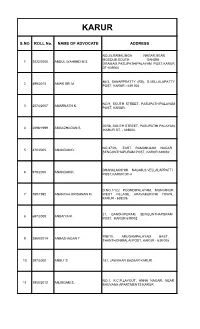
S.NO ROLL No. NAME of ADVOCATE ADDRESS
KARUR S.NO ROLL No. NAME OF ADVOCATE ADDRESS NO:25,RAMALINGA NAGAR,NEAR MOSQUE,SOUTH GANDHI 1 2022/2000 ABDUL WAHEED M.S. GRAMAM,PASUPATHIPALAYAM POST,KARUR DT-639004 86/3, SANAPPRATTY (RS), S.VELLALAPATTY 2 499/2015 AMAR SRI M. POST, KARUR - 639 004 NO.9, SOUTH STREET, PASUPATHIPALAYAM 3 2574/2007 AMARNATH K. POST, KARUR. 20/58, SOUTH STREET, PASUPATHI PALAYAM, 4 2098/1999 AMBAZHAGAN S. KARUR DT. - 639004. NO.37/26, EAST RAMANUJAM NAGAR 5 470/2005 ANANDAN D. SENGUNTHAPURAM POST, KARUR 639002 DHANALAKSHMI NAGAR,S.VELLALAPPATTI 6 979/2000 ANANDAN K. POST,KARUR DT-4 D.NO.1/122 POONDIPALAYAM, MONJANUR 7 789/1992 ANANTHA KRISHNAN M. WEST VILLAGE, ARAVAKURICHI TOWN, KARUR - 639206. 21, GANDHIPURAM, SENGUNTHAPURAM 8 697/2009 ANBAIYA R. POST, KARUR 639002 70E/10, ARUGAMPALAYAM EAST, 9 2869/2014 ANBAZHAGAN T. THANTHONIMALAI POST, KARUR - 639 005 10 297/2000 ANBU S. 181, JAWAHAR BAZAAR KARUR. NO.1, K.C.P.LAYOUT, ANNA NAGAR, NEAR 11 1950/2010 ANJUGAM S. BHUVANA APARTMENTS KARUR S.NO ROLL No. NAME OF ADVOCATE ADDRESS AVUTHIPALAYAM, PAGANATHAM PO, KARUR 12 2898/2009 ANNADURAI A. 639005 NO.11, 3RD STREET, JEEVA NAGAR, 13 454/2003 ANNADURAI V.M. VENGAMEDU KARUR -639006 14 976/2006 ARASILANG KUMARAVEL A. 2/84, MELAPALAYAM PO, KARUR 639114 NO:3/79, AMBETKAR NAGAR PANAYAM 15 850/2013 ARASU D. PALAYAM, GUDALORE POST, THENNLEI VIA, ARAVI TALUK, KARUR DIST - 639 206 34/40A, GANDHI NAGAR, 3RD STREET 16 1632/2014 ARIKARAN R. VELAYUTHAMPALAYAM POST, KARUR NO.3, PALANI RAMU ILLAM, M.G.R.NAGAR, 17 605/2011 ARIVUMANI G. -

District Survey Report Karur District
DISTRICT SURVEY REPORT KARUR DISTRICT DISTRICT ENVIRONMENT IMPACT ASSESSMENT AUTHORITY (DEIAA), KARUR SEPTEMBER --- 2017 1 DIRECTORATE OF GEOLOGY AND MINING MINERAL RESOURCE DEPARTMENT GOVERNMENT OF TAMILNADU DISTRICT SURVEY REPORT KARUR S.No Content Page No 1 Introduction 1-5 1.1 Physiographic 6 1.2 Location & accessibility 6 1.3 Soil 6 1.4 Geomorphology 7-8 2 GEOLOGY 8-9 3 Land utilization pattern in Karur District 9-10 4 Month wise Rainfall details in Karur Dis trict for the last three years. 10-11 5 Groundwater Potential of Karur District 11-12 Over view of Mining Activity in the District 6 12-21 Granite 6.1 12-13 6.2 Limestone 14 6.3 Quartz and Feldspar 15-16 Roughstone 6.4 16-18 6.5 Dunite Querry 18 6.6 Gemstones 19 6.7 Sand 20-21 List of Annexure Annexure:I The list of mining leases in the district with location, area and period of validity Annexure:II Details of royalty or revenue received in the last there years Annexure:III Detail of production of sand or bajari or mino r mineral in last three years 2 Introduction Karur district lies between 10 °63' and 11°14 ' north latitude and77 °90' and 78° 61' east longitude. Karur town is located on the bank of Amaravathi river. Karur is one of the oldest towns in the State of Tamil Nadu and has played a very significant role in the history and culture of the Tamils. Its history dates back over 2000 years, and has been a flourishing trading centre even in the early Sangam days.