ECOLOGICAL ADAPTATIONS of the FLORAL STRUCTURES of Galanthus Nivalis L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE
Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE LILIACEAE de Jussieu 1789 (Lily Family) (also see AGAVACEAE, ALLIACEAE, ALSTROEMERIACEAE, AMARYLLIDACEAE, ASPARAGACEAE, COLCHICACEAE, HEMEROCALLIDACEAE, HOSTACEAE, HYACINTHACEAE, HYPOXIDACEAE, MELANTHIACEAE, NARTHECIACEAE, RUSCACEAE, SMILACACEAE, THEMIDACEAE, TOFIELDIACEAE) As here interpreted narrowly, the Liliaceae constitutes about 11 genera and 550 species, of the Northern Hemisphere. There has been much recent investigation and re-interpretation of evidence regarding the upper-level taxonomy of the Liliales, with strong suggestions that the broad Liliaceae recognized by Cronquist (1981) is artificial and polyphyletic. Cronquist (1993) himself concurs, at least to a degree: "we still await a comprehensive reorganization of the lilies into several families more comparable to other recognized families of angiosperms." Dahlgren & Clifford (1982) and Dahlgren, Clifford, & Yeo (1985) synthesized an early phase in the modern revolution of monocot taxonomy. Since then, additional research, especially molecular (Duvall et al. 1993, Chase et al. 1993, Bogler & Simpson 1995, and many others), has strongly validated the general lines (and many details) of Dahlgren's arrangement. The most recent synthesis (Kubitzki 1998a) is followed as the basis for familial and generic taxonomy of the lilies and their relatives (see summary below). References: Angiosperm Phylogeny Group (1998, 2003); Tamura in Kubitzki (1998a). Our “liliaceous” genera (members of orders placed in the Lilianae) are therefore divided as shown below, largely following Kubitzki (1998a) and some more recent molecular analyses. ALISMATALES TOFIELDIACEAE: Pleea, Tofieldia. LILIALES ALSTROEMERIACEAE: Alstroemeria COLCHICACEAE: Colchicum, Uvularia. LILIACEAE: Clintonia, Erythronium, Lilium, Medeola, Prosartes, Streptopus, Tricyrtis, Tulipa. MELANTHIACEAE: Amianthium, Anticlea, Chamaelirium, Helonias, Melanthium, Schoenocaulon, Stenanthium, Veratrum, Toxicoscordion, Trillium, Xerophyllum, Zigadenus. -

CULTURAL HERITAGE in MIGRATION Published Within the Project Cultural Heritage in Migration
CULTURAL HERITAGE IN MIGRATION Published within the project Cultural Heritage in Migration. Models of Consolidation and Institutionalization of the Bulgarian Communities Abroad funded by the Bulgarian National Science Fund © Nikolai Vukov, Lina Gergova, Tanya Matanova, Yana Gergova, editors, 2017 © Institute of Ethnology and Folklore Studies with Ethnographic Museum – BAS, 2017 © Paradigma Publishing House, 2017 ISBN 978-954-326-332-5 BULGARIAN ACADEMY OF SCIENCES INSTITUTE OF ETHNOLOGY AND FOLKLORE STUDIES WITH ETHNOGRAPHIC MUSEUM CULTURAL HERITAGE IN MIGRATION Edited by Nikolai Vukov, Lina Gergova Tanya Matanova, Yana Gergova Paradigma Sofia • 2017 CONTENTS EDITORIAL............................................................................................................................9 PART I: CULTURAL HERITAGE AS A PROCESS DISPLACEMENT – REPLACEMENT. REAL AND INTERNALIZED GEOGRAPHY IN THE PSYCHOLOGY OF MIGRATION............................................21 Slobodan Dan Paich THE RUSSIAN-LIPOVANS IN ITALY: PRESERVING CULTURAL AND RELIGIOUS HERITAGE IN MIGRATION.............................................................41 Nina Vlaskina CLASS AND RELIGION IN THE SHAPING OF TRADITION AMONG THE ISTANBUL-BASED ORTHODOX BULGARIANS...............................55 Magdalena Elchinova REPRESENTATIONS OF ‘COMPATRIOTISM’. THE SLOVAK DIASPORA POLITICS AS A TOOL FOR BUILDING AND CULTIVATING DIASPORA.............72 Natália Blahová FOLKLORE AS HERITAGE: THE EXPERIENCE OF BULGARIANS IN HUNGARY.......................................................................................................................88 -

BURIED TREASURE Summer 2019 Rannveig Wallis, Llwyn Ifan, Porthyrhyd, Carmarthen, UK
BURIED TREASURE Summer 2019 Rannveig Wallis, Llwyn Ifan, Porthyrhyd, Carmarthen, UK. SA32 8BP Email: [email protected] I am still trying unsuccessfully to retire from this enterprise. In order to reduce work, I am sowing fewer seeds and concentrating on selling excess stock which has been repotted in the current year. Some are therefore in quite small numbers. I hope that you find something of interest and order early to avoid any disappointments. Please note that my autumn seed list is included below. This means that seed is fresher and you can sow it earlier. Terms of Business: I can accept payment by either: • Cheque made out to "R Wallis" (n.b. Please do not fill in the amount but add the words “not to exceed £xx” ACROSS THE TOP); • PayPal, please include your email address with the order and wait for an invoice after I dispatch your order; • In cash (Sterling, Euro or US dollar are accepted, in this case I advise using registered mail). Please note that I can only accept orders placed before the end of August. Parcels will be dispatched at the beginning of September. If you are going to be away please let me know so that I can coordinate dispatch. I will not cash your cheque until your order is dispatched. If ordering by email, and following up by post, please ensure that you tick the box on the order form to avoid duplication. Acis autumnalis var pulchella A Moroccan version of this excellent early autumn flowerer. It is quite distinct in the fact that the pedicels and bracts are green rather than maroon as in the type variety. -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Conserving Europe's Threatened Plants
Conserving Europe’s threatened plants Progress towards Target 8 of the Global Strategy for Plant Conservation Conserving Europe’s threatened plants Progress towards Target 8 of the Global Strategy for Plant Conservation By Suzanne Sharrock and Meirion Jones May 2009 Recommended citation: Sharrock, S. and Jones, M., 2009. Conserving Europe’s threatened plants: Progress towards Target 8 of the Global Strategy for Plant Conservation Botanic Gardens Conservation International, Richmond, UK ISBN 978-1-905164-30-1 Published by Botanic Gardens Conservation International Descanso House, 199 Kew Road, Richmond, Surrey, TW9 3BW, UK Design: John Morgan, [email protected] Acknowledgements The work of establishing a consolidated list of threatened Photo credits European plants was first initiated by Hugh Synge who developed the original database on which this report is based. All images are credited to BGCI with the exceptions of: We are most grateful to Hugh for providing this database to page 5, Nikos Krigas; page 8. Christophe Libert; page 10, BGCI and advising on further development of the list. The Pawel Kos; page 12 (upper), Nikos Krigas; page 14: James exacting task of inputting data from national Red Lists was Hitchmough; page 16 (lower), Jože Bavcon; page 17 (upper), carried out by Chris Cockel and without his dedicated work, the Nkos Krigas; page 20 (upper), Anca Sarbu; page 21, Nikos list would not have been completed. Thank you for your efforts Krigas; page 22 (upper) Simon Williams; page 22 (lower), RBG Chris. We are grateful to all the members of the European Kew; page 23 (upper), Jo Packet; page 23 (lower), Sandrine Botanic Gardens Consortium and other colleagues from Europe Godefroid; page 24 (upper) Jože Bavcon; page 24 (lower), Frank who provided essential advice, guidance and supplementary Scumacher; page 25 (upper) Michael Burkart; page 25, (lower) information on the species included in the database. -

Review of Species Selected from the Analysis of 2004 EC Annual Report
Review of species selected from the Analysis of 2005 EC Annual Report to CITES (Version edited for public release) Prepared for the European Commission Directorate General E - Environment ENV.E.2. – Development and Environment by the United Nations Environment Programme World Conservation Monitoring Centre May, 2008 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK ABOUT UNEP WORLD CONSERVATION MONITORING CENTRE www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme (UNEP), the world‘s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision- makers recognize the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre‘s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. The designations employed and the presentations do not imply the expressions of any opinion whatsoever on the part of UNEP, the European Commission or contributory organisations concerning the legal status of any country, territory, city or area or its authority, or concerning the delimitation of its frontiers or boundaries. -

Subsection from Buna to Počitelj - 7.2 Km
BiodiversityAssessment: Corridor Vc2 Project, Federation of Bosnia and Herzegovina (FBiH) - subsection from Buna to Počitelj - 7.2 km - March 2016 1 Name: BiodiversityAssessment: Corridor Vc2 Project, Federation of Bosnia and Herzegovina (FBiH) - subsection from Buna to Počitelj - 7.2 km - Investor: IPSA INSTITUT doo Put života bb 71000 SARAJEVO Language: English Contractor: Center for economic, technological and environmental development – CETEOR doo Sarajevo Topal Osman Paše 32b BA, 71000 Sarajevo Phone:+ 387 33 563 580 Fax: +387 33 205 725 E-mail: [email protected] with: Doc. Dr Samir Đug Mr Sc Nusret Drešković, Date: Mart 2016 Number: 01/P-1478/14 2 1. Flora and Vegetation The southern part of Herzegovina where is situated the alignment is distinguished by the presence of mainly evergreen vegetation with numerous typical Mediterranean plants and rich fauna. 1.1. Flora The largest number of plant species belongs to the various subgroups of Mediterranean floral element. The most abundant plant families are grasses (Poaceae), legumes (Fabaceae) and aster family (Asteraceae), which also indicates Mediterranean and Submediterranean features of the flora. After literature data (Šilić 1996), some 10% of rare, endangered and endemic plant species from Bosnia and Herzegovina grows in Mediterranean region of the country. From this list, in investigated area could be found vulnerable species Celtis tournefortii, Cyclamen neapolitanum, Cyclamen repandum, Acanthus spinossisimus, Ruscus aculeatus, Galanthus nivalis, Orchis simia, Orchis spitzelii and some others. Rare species in this area are: Dittrichia viscosa, Rhamnus intermedius, Petteria ramentacea, Moltkia petraea, and Asphodelus aestivus. By the roads and in the areas under strong human impacts could be found certain introduced species, such as Paspalum paspaloides, P. -

TELOPEA Publication Date: 13 October 1983 Til
Volume 2(4): 425–452 TELOPEA Publication Date: 13 October 1983 Til. Ro)'al BOTANIC GARDENS dx.doi.org/10.7751/telopea19834408 Journal of Plant Systematics 6 DOPII(liPi Tmst plantnet.rbgsyd.nsw.gov.au/Telopea • escholarship.usyd.edu.au/journals/index.php/TEL· ISSN 0312-9764 (Print) • ISSN 2200-4025 (Online) Telopea 2(4): 425-452, Fig. 1 (1983) 425 CURRENT ANATOMICAL RESEARCH IN LILIACEAE, AMARYLLIDACEAE AND IRIDACEAE* D.F. CUTLER AND MARY GREGORY (Accepted for publication 20.9.1982) ABSTRACT Cutler, D.F. and Gregory, Mary (Jodrell(Jodrel/ Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey, England) 1983. Current anatomical research in Liliaceae, Amaryllidaceae and Iridaceae. Telopea 2(4): 425-452, Fig.1-An annotated bibliography is presented covering literature over the period 1968 to date. Recent research is described and areas of future work are discussed. INTRODUCTION In this article, the literature for the past twelve or so years is recorded on the anatomy of Liliaceae, AmarylIidaceae and Iridaceae and the smaller, related families, Alliaceae, Haemodoraceae, Hypoxidaceae, Ruscaceae, Smilacaceae and Trilliaceae. Subjects covered range from embryology, vegetative and floral anatomy to seed anatomy. A format is used in which references are arranged alphabetically, numbered and annotated, so that the reader can rapidly obtain an idea of the range and contents of papers on subjects of particular interest to him. The main research trends have been identified, classified, and check lists compiled for the major headings. Current systematic anatomy on the 'Anatomy of the Monocotyledons' series is reported. Comment is made on areas of research which might prove to be of future significance. -

CZECH REPUBLIC: COUNTRY REPORT to the FAO INTERNATIONAL TECHNICAL CONFERENCE on PLANT GENETIC RESOURCES (Leipzig 1996)
CZECH REPUBLIC: COUNTRY REPORT TO THE FAO INTERNATIONAL TECHNICAL CONFERENCE ON PLANT GENETIC RESOURCES (Leipzig 1996) Prepared by: Ladislav Dotlac˘il Karel Vanc˘ura Prague, April 1995 BANGLADESH country report 2 Note by FAO This Country Report has been prepared by the national authorities in the context of the preparatory process for the FAO International Technical Conference on Plant Genetic Resources, Leipzig, Germany, 17-23 June 1996. The Report is being made available by FAO as requested by the International Technical Conference. However, the report is solely the responsibility of the national authorities. The information in this report has not been verified by FAO, and the opinions expressed do not necessarily represent the views or policy of FAO. The designations employed and the presentation of the material and maps in this document do not imply the expression of any option whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. BANGLADESH country report 3 Table of Contents CHAPTER 1 CHARACTERISTICS OF THE CZECH REPUBLIC, ITS AGRICULTURAL AND FORESTRY SECTORS 6 1.1 GENERAL INFORMATION 6 1.2 AGRICULTURE IN THE CZECH REPUBLIC 8 1.3 FORESTRY IN THE CZECH REPUBLIC 10 CHAPTER 2 INDIGENOUS PLANT GENETIC RESOURCES 12 2.1 FOREST GENETIC RESOURCES 12 2.2 WILD SPECIES AND WILD RELATIVES OF CROP PLANTS 13 2.3 LANDRACES (FARMERS‘ VARIETIES) AND OLD CULTIVARS 15 CHAPTER -

Neretva and Trebišnjica River Basin (NTRB)
E1468 Consulting Services for Environment Impact Assessment Public Disclosure Authorized in the Neretva and Trebišnjica River Basin (NTRB) No. TF052845/GE-P084608 Public Disclosure Authorized F I N A L EIA R E P O R T Public Disclosure Authorized Public Disclosure Authorized Sarajevo/Banja Luka, August 2006 Bosnia and Herzegovina and Croatia Proposed Integrated Ecosystem Management of the Nerteva and Trebišnjica River Basin (NTRB) Project Table of Contents Abbreviations and Acronyms EXECUTIVE SUMMARY List of Tables List of Pictures List of Annexes References 1. PROJECT DESCRIPTION .....................................................................................14 1.1. Background .............................................................................................. 14 1.2. Project objectives..................................................................................... 15 1.3. Project components ................................................................................. 16 2. POLICY, LEGAL AND ADMINISTRATIVE FRAMEWORK ......................................21 2.1. Overall Project Implementation Arrangements....................................... 21 2.2. Requirements of the WB .......................................................................... 22 2.3. Bosnia and Herzegovina environmental policy ........................................ 23 2.4. Legislation of Republic of Croatia ............................................................ 26 2.5. Evaluation of project environmental aspects .................................................27 -
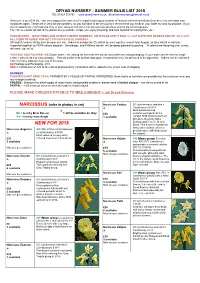
Dryad Nursery, SUMMER 2018 Bulb List
DRYAD NURSERY - SUMMER BULB LIST 2018 TEL 01423 358791 www.dryad-home.co.uk Email [email protected] Welcome to my 2018 list. I am very happy to be able to offer a particularly good number of famous Irish breeder Brian Duncan's very desirable new miniatures again. These are in very limited numbers, so you will have to be very quick! I recommend you email in your order as soon as possible. If you wish to telephone, I will note the time of your request and slot it into the appropriate place among the email requests. You can see better photos of the plants on my website. I hope you enjoy browsing, and look forward to hearing from you. PLEASE NOTE – MOST ITEMS ARE IN VERY LIMITED NUMBERS, SO PLEASE APPLY EARLY, LAST DATE FOR ORDERS END OF JULY 2017 ALL ITEMS OFFERED SUBJECT TO SUCCESSFUL HARVEST. UK and EU orders will be sent as soon as I can. Orders to outside the EU will all be sent together end of July/ August, as they will all need to be inspected together by FERA before dispatch. Snowdrops, and Fritillaria davidii will be damp-packed for posting. All plants are flowering size, unless otherwise specified. POSTAGE AND PACKING- -£5-50 per order. I am asking for less than the actual cost of delivery and packaging. If your order can be sent as Large Letter, I will refund any extra postage. Parcels will be sent by first class post, recorded delivery, so will need to be signed for. Orders can be collected from my home address if you are in the area. -

Flora Mediterranea 26
FLORA MEDITERRANEA 26 Published under the auspices of OPTIMA by the Herbarium Mediterraneum Panormitanum Palermo – 2016 FLORA MEDITERRANEA Edited on behalf of the International Foundation pro Herbario Mediterraneo by Francesco M. Raimondo, Werner Greuter & Gianniantonio Domina Editorial board G. Domina (Palermo), F. Garbari (Pisa), W. Greuter (Berlin), S. L. Jury (Reading), G. Kamari (Patras), P. Mazzola (Palermo), S. Pignatti (Roma), F. M. Raimondo (Palermo), C. Salmeri (Palermo), B. Valdés (Sevilla), G. Venturella (Palermo). Advisory Committee P. V. Arrigoni (Firenze) P. Küpfer (Neuchatel) H. M. Burdet (Genève) J. Mathez (Montpellier) A. Carapezza (Palermo) G. Moggi (Firenze) C. D. K. Cook (Zurich) E. Nardi (Firenze) R. Courtecuisse (Lille) P. L. Nimis (Trieste) V. Demoulin (Liège) D. Phitos (Patras) F. Ehrendorfer (Wien) L. Poldini (Trieste) M. Erben (Munchen) R. M. Ros Espín (Murcia) G. Giaccone (Catania) A. Strid (Copenhagen) V. H. Heywood (Reading) B. Zimmer (Berlin) Editorial Office Editorial assistance: A. M. Mannino Editorial secretariat: V. Spadaro & P. Campisi Layout & Tecnical editing: E. Di Gristina & F. La Sorte Design: V. Magro & L. C. Raimondo Redazione di "Flora Mediterranea" Herbarium Mediterraneum Panormitanum, Università di Palermo Via Lincoln, 2 I-90133 Palermo, Italy [email protected] Printed by Luxograph s.r.l., Piazza Bartolomeo da Messina, 2/E - Palermo Registration at Tribunale di Palermo, no. 27 of 12 July 1991 ISSN: 1120-4052 printed, 2240-4538 online DOI: 10.7320/FlMedit26.001 Copyright © by International Foundation pro Herbario Mediterraneo, Palermo Contents V. Hugonnot & L. Chavoutier: A modern record of one of the rarest European mosses, Ptychomitrium incurvum (Ptychomitriaceae), in Eastern Pyrenees, France . 5 P. Chène, M.