Ecological Status of Hot Springs in Eastern Amhara Region: Macroinvertebrates Diversity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Districts of Ethiopia
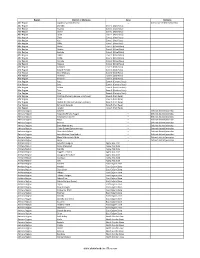
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

Evaluation of Measles Outbreak Response Activities And
omens H OPEN ACCESS Freely available online f W ea o l l th a n C r a u r e o J Journal of Women’s Health Care ISSN: 2167-0420 Research Article Evaluation of Measles Outbreak Response Activities and Surveillance System Performance in Nunukumba District, East Wollega Zone of Oromia Region, Ethiopia, June 2020 Zalalem Kaba Babure1*, Aklilu Fikadu Tufa2 1East Wollega Zonal Health Office, Nekemte Town, Oromia Regional State, Western Ethiopia; 2Primary Health Care Project, East Wollega Zone, Nekemte, Ethiopia ABSTRACT Background: Measles is one of the world’s most contagious viral diseases that have the potential to be life-threatening. In Ethiopia, measles is among the most common cause for morbidity and mortality in children. Major outbreaks with large attack rates resulting in as high as 15-20% case fatality rates have been reported in this country. There is a paucity of information on measles outbreak response activities and surveillance system performance in Eats Wollega Zone in general and NunuKumba district in particular, Ethiopia. Methods: A descriptive cross-sectional survey was employed from June 01-05, 2020 at NunuKumba district of East Wollega Zone. The data was collected by three senior technical experts using semi-structured questionnaires, and a secondary data was taken from the line list of cases. Purposive sampling technique was used. Quantitative data was analyzed using Microsoft Excel 2010 while the qualitative data was themed; analyzed and then triangulated with quantitative result. The result was displayed by graphs, tables and Epi-curve. Results: The overall Attack rate and Case Fatality Rate were 1.05% and 0.41% respectively. -

English-Full (0.5
Enhancing the Role of Forestry in Building Climate Resilient Green Economy in Ethiopia Strategy for scaling up effective forest management practices in Amhara National Regional State with particular emphasis on smallholder plantations Wubalem Tadesse Alemu Gezahegne Teshome Tesema Bitew Shibabaw Berihun Tefera Habtemariam Kassa Center for International Forestry Research Ethiopia Office Addis Ababa October 2015 Copyright © Center for International Forestry Research, 2015 Cover photo by authors FOREWORD This regional strategy document for scaling up effective forest management practices in Amhara National Regional State, with particular emphasis on smallholder plantations, was produced as one of the outputs of a project entitled “Enhancing the Role of Forestry in Ethiopia’s Climate Resilient Green Economy”, and implemented between September 2013 and August 2015. CIFOR and our ministry actively collaborated in the planning and implementation of the project, which involved over 25 senior experts drawn from Federal ministries, regional bureaus, Federal and regional research institutes, and from Wondo Genet College of Forestry and Natural Resources and other universities. The senior experts were organised into five teams, which set out to identify effective forest management practices, and enabling conditions for scaling them up, with the aim of significantly enhancing the role of forests in building a climate resilient green economy in Ethiopia. The five forest management practices studied were: the establishment and management of area exclosures; the management of plantation forests; Participatory Forest Management (PFM); agroforestry (AF); and the management of dry forests and woodlands. Each team focused on only one of the five forest management practices, and concentrated its study in one regional state. -

Periodic Monitoring Report Working 2016 Humanitarian Requirements Document – Ethiopia Group
DRMTechnical Periodic Monitoring Report Working 2016 Humanitarian Requirements Document – Ethiopia Group Covering 1 Jan to 31 Dec 2016 Prepared by Clusters and NDRMC Introduction The El Niño global climactic event significantly affected the 2015 meher/summer rains on the heels of failed belg/ spring rains in 2015, driving food insecurity, malnutrition and serious water shortages in many parts of the country. The Government and humanitarian partners issued a joint 2016 Humanitarian Requirements Document (HRD) in December 2015 requesting US$1.4 billion to assist 10.2 million people with food, health and nutrition, water, agriculture, shelter and non-food items, protection and emergency education responses. Following the delay and erratic performance of the belg/spring rains in 2016, a Prioritization Statement was issued in May 2016 with updated humanitarian requirements in nutrition (MAM), agriculture, shelter and non-food items and education.The Mid-Year Review of the HRD identified 9.7 million beneficiaries and updated the funding requirements to $1.2 billion. The 2016 HRD is 69 per cent funded, with contributions of $1.08 billion from international donors and the Government of Ethiopia (including carry-over resources from 2015). Under the leadership of the Government of Ethiopia delivery of life-saving and life- sustaining humanitarian assistance continues across the sectors. However, effective humanitarian response was challenged by shortage of resources, limited logistical capacities and associated delays, and weak real-time information management. This Periodic Monitoring Report (PMR) provides a summary of the cluster financial inputs against outputs and achievements against cluster objectives using secured funding since the launch of the 2016 HRD. -

Pdf | 418.64 Kb
ETHIOPIA Food Security Outlook Update November 2010 Poor water availability in the southeast likely to follow below-average Oct-Dec rains Key Messages Figure 1. Estimated food security outcomes, October to December 2010 To date, the performance of bega/hageya/deyr rains has been below average as predicted. This has resulted in shortages of pasture and water in the southeastern pastoral and agropastoral parts of the country. Land preparation and planting of transitional crops, mainly sweet potato has been carried out as usual in SNNPR. Performance of these crops will highly depend on the performance of the sapie rains in December that are predicted to be normal to below normal this year. Overall meher season crop harvests (October to January) are expected to be normal to above normal this year, except in areas that were affected by water logging, floods, and yellow wheat rust, resulting in an overall improvement in food security in dominantly meher crop producing parts Source: FEWS NET and WFP of the country. Figure 2. Estimated food security outcomes, January to March 2010 Updated food security outlook through March 2011 The period November to March is typically a time of stable food security in Ethiopia given the meher harvest (October to January) which contributes 90 to 95 percent of total annual crop production. With the start of the harvest, staple food prices typically decline, further contributing to the improvement of food security. This year, the overall performance of meher season crops has been average to above average, although localized shocks led to reductions in production in some areas. -

Ethiopia Humanitarian Fund 2016 Annual Report
2016 Annual Report Ethiopia Humanitarian Fund Ethiopia Humanitarian Fund 2016 Annual Report TABLE of CONTENTS Forward by the Humanitarian Coordinator 04 Dashboard – Visual Overview 05 Humanitarian Context 06 Allocation Overview 07 Fund Performance 09 Donor Contributions 12 Annexes: Summary of results by Cluster Map of allocations Ethiopia Humanitarian Fund projects funded in 2016 Acronyms Useful Links 1 REFERENCE MAP N i l e SAUDI ARABIA R e d ERITREA S e a YEMEN TIGRAY SUDAN Mekele e z e k e T Lake Tana AFAR DJIBOUTI Bahir Dar Gulf of Aden Asayita AMHARA BENESHANGUL Abay GUMU Asosa Dire Dawa Addis Ababa Awash Hareri Ji Jiga Gambela Nazret (Adama) GAMBELA A EETHIOPIAT H I O P I A k o b o OROMIA Awasa Omo SOMALI SOUTH S SNNPR heb SUDAN ele le Gena Ilemi Triangle SOMALIA UGANDA KENYA INDIAN OCEAN 100 km National capital Regional capital The boundaries and names shown and the designations International boundary used on this map do not imply official endorsement or Region boundary acceptance by the United Nations. Final boundary River between the Republic of Sudan and the Republic of Lake South Sudan has not yet been determined. 2 I FOREWORD DASHBOARD 3 FOREWORD FOREWORD BY THE HUMANITARIAN COORDINATOR In 2016, Ethiopia continued to battle the 2015/2016 El Niño-induced drought; the worst drought to hit the country in fifty years. More than 10.2 million people required relief food assistance at the peak of the drought in April. To meet people’s needs, the Government of Ethiopia and humanitar- ian partners issued an initial appeal for 2016 of US$1.4 billion, which increased to $1.6 billion in August. -

Compiled Body of Works
Compiled Body of Works Addis Ababa University, College of Health Sciences, School of Public Health Ethiopia Field Epidemiology Training Program (EFETP) Compiled Body of Works in Field Epidemiology By Ashenafi Ayalew Submitted to the school of Graduate Studies of Addis Ababa University in partial fulfillment for Degree of Masters of Public Health in Field Epidemiology May 1, 2015 Addis Ababa Ashenafi,[email protected] i Compiled Body of Works Addis Ababa University College of Health Sciences School of Public Health Ethiopia Field Epidemiology Training Program (EFETP) Compiled Body of Works in Field Epidemiology By Ashenafi Ayalew Worku Submitted to the School of Graduate Studies of Addis Ababa University in partial Fulfillment for the degree of Master of Public Health in Field Epidemiology Advisors 1-Dr Fikre Enquoselassie (Ph.D.) 2-Mr. Addisu Workineh (B.sc, M.P.H) May 1, 2015 Addis Ababa Ashenafi,[email protected] ii Compiled Body of Works ADDIS ABABA UNIVERSITY School of Graduate Studies Compiled Body of Works in Field Epidemiology By Ashenafi Ayalew Worku Field Epidemiology and Laboratory Training Program (EFELTP) School of Public Health, College of Health Sciences Addis Ababa University Approval by Examining Board _________________________ ___________________ Chairman, School Graduate Committee _________________________ ___________________ Advisor _________________________ ___________________ Examiner _________________________ ___________________ Examiner Ashenafi,[email protected] iii Compiled Body of Works Acknowledgement First and foremost, I am very grateful to the God for giving me the health, wellbeing and the strength that were very fundamental for the successful accomplishment of this venture. I am also grateful to my mentors Dr. Fikre Enquoselassie and Mr. Addisu Workineh for their support and guidance while I was working my outputs. -

The Development Study on the Improvement of Livelihood Through Integrated Watershed Management in Amhara Region
Food Security Coordination & Disaster Prevention Office Bureau of Agriculture and Rural Development The Government of Amhara National Regional State The Federal Democratic Republic of Ethiopia THE DEVELOPMENT STUDY ON THE IMPROVEMENT OF LIVELIHOOD THROUGH INTEGRATED WATERSHED MANAGEMENT IN AMHARA REGION FINAL REPORT March 2011 Japan International Cooperation Agency SANYU CONSULTANTS INC. ETO JR 11-002 Location map Location Map of the Study Area FINAL REPORT Summary 1. INTRODUCTION OF THE STUDY Amhara National Regional State (ANRS) located in the northern part of the Federal Democratic Republic of Ethiopia has the area of about 154 thousand sq. km. accounting for around 15% of the territory of the nation where about 17.9 million (or about 25% of the national population) are inhabited. In the ANRS, 6.3 million people are considered as food insecure. In particular, the eastern area of Amhara Region has been exposed to recurrent drought for past 3 decades, thus considered as the most suffering area from food shortage. Under such circumstances, the Federal Government of Ethiopia requested cooperation of Japan for “The Development Study on the Improvement of Livelihood through Integrated Watershed Management in Amhara Region”. Based on this request, Japan International Cooperation Agency (JICA) dispatched a preparatory study team to Ethiopia in March 2007 and agreed the basic contents of Scope of Work (S/W) that were signed between the ANRS Government and JICA in the same month. Under such background, JICA dispatched a Study Team to the ANRS from March 2008 to conduct the Study as described in the S/W. The overall goal of the Study is to ensure food security of the vulnerable households in food insecure area of Amhara Region through implementation of integrated watershed management. -

Hc Bulletin April May 2021 V1.Pdf
HEALTH CLUSTER BULLETIN #23 Group counselling session by IOM MHPSS staffs in Mekelle IDP sites, April & May 2021 May 2021 Photo by: IOM. Ethiopia Emergency type: Multiple Events Reporting period: 1-30 April & 1-31 May 8.7 M 2.9 M 1.6 M 220 PEOPLE IN NEED IDP TARGETED HOST TARGETED WOREDAS HIGHLIGHTS HEALTH SECTOR • The health cluster require US$48.2M to HEALTH CLUSTER 30 IMPLEMENTING support partners sustain operations in PARTNERS Tigray region for the next 8 months (From MEDICINES DELIVERED TO HEALTH May to December 2021). FACILITIES/PARTNERS • Healh cluster is targeting 2.3M out of the 751 ASSORTED MEDICAL KITS estimated 3.8M people in need of health HEALTH CLUSTER ACTIVITIES care services in Tigray. • From January to May 2021 the health 394,693 OPD CONSULTATIONS cluster reached a cumulative of total of 1,421,169 people with health IEC messages. VACCINATION VACCINATED AGAINST 9,040 MEASLES • Majority of IDPs live in host communities (over 90%),only a small number live in EWARS sites. North Shewa has 253,000 IDPs and CONFIRMED COVID-19, POLIO, YELLOW FEVER, Oromia S. zone 105,000; the IDPs need 5 food, ES/NFIs, WASH and healthcare. CHOLERA, MEASLES OUTBREAKS FUNDING $US • As of 23 May 2021, Ethiopia recorded 140 M REQUESTED 269,194 confirmed COVID 19 cases,with M % FUNDED 4,076 deaths and 228,757 recoveries. M GAP have been reported in Ethiopia. 1 Situation update The situation in the Tigray region was becoming protracted with increased humanitarian needs. Hence, the available resources in the country would be insufficient to meet the growing need to rapidly respond to the public health consequences of the conflict. -

Summary and Statistical Report of the 2007 Population and Housing Census Results
Summary and Statistical Report of the 2007 Population and Housing Census Results Summary and Statistical Report of the 2007 Population and Housing Census Results 1 2 Summary and Statistical Report of the 2007 Population and Housing Census Results This document was printed by United Nations Population Fund(UNFPA) Summary and Statistical Report of the 2007 Population and Housing Census Results Summary and Statistical Report of the 2007 Population and Housing Census Results 3 Acknowledgements The third Population and Housing Census of Ethiopia was conducted in May and Novem- ber 2007. In accordance with the Census Commission Re-establishment Proclamation, the House of Peoples Representatives endorsed, on 4 December 2008, the census findings as organized by age, sex, religion, ethnicity, region and urban and rural residence. This sum- mary report contains an extract of the census data from this first release and the remaining census results will be disseminated subsequently. The Population and Housing Census which is a huge, complex and costly statistical opera- tion has been successfully completed through the concerted efforts of different government and non-government organizations, development partners, and individuals. The Office of the Census Commission and the Central Statistical Agency would, therefore, like to thank all who have contributed to the successful completion of the census. The Office of the Census Commission is very grateful to the Government of Ethiopia for its huge financial and administrative support. The Office is grateful to development partners particularly the United Nations Population Fund (UNFPA) and the Department for International Development (DfID) for their generous financial, logistics and technical support. -

Periodic Monitoring Report Jan-Sep 2016 Final.Pdf (Английский (English))
DRMTechnical Periodic Monitoring Report Working 2016 Humanitarian Requirements Document – Ethiopia Group Covering 1 Jan to 30 Sep 2016 Prepared by Clusters and NDRMC Introduction The El Niño global climactic event significantly affected the 2015 meher/summer rains on the heels of failed belg/ spring rains in 2015, driving food insecurity, malnutrition and serious water shortages in many parts of the country. The Government and humanitarian partners issued a joint 2016 Humanitarian Requirements Document (HRD) in December 2015 requesting US$1.4 billion to assist 10.2 million people with food, health and nutrition, water, agriculture, shelter and non-food items, protection and emergency education responses. Following the delay and erratic performance of the belg/spring rains in 2016, a Prioritization Statement was issued in May 2016 with updated humanitarian requirements in nutrition (MAM), agriculture, shelter and non-food items and education.The Mid-Year Review of the HRD identified 9.7 million beneficiaries and updated the funding requirements to US$1.2 billion. As of 30 September, the HRD is 65 per cent funded, with contributions of US$1.06 billion from international donors and the Government of Ethiopia (including carry-over resources from 2015). Under the leadership of the Government of Ethiopia delivery of life-saving and life-sustaining humanitarian assistance continues across the sectors. However, effective humanitarian response was challenged by shortage of resources, limited logistical capacities and associated delays, and weak real-time information management. This Periodic Monitoring Report (PMR) provides a summary of the cluster financial inputs against outputs and achievements against cluster objectives using secured funding since the launch of the 2016 HRD. -

Characterization of Goat and Sheep Production System in Oromia Zone of Amhara Region, Ethiopia
European Journal of Applied Sciences 11 (3): 83-102, 2019 ISSN 2079-2077 © IDOSI Publications, 2019 DOI: 10.5829/idosi.ejas.2019.83.102 Characterization of Goat and Sheep Production System in Oromia Zone of Amhara Region, Ethiopia 1Teshome Mulualem, 23Ahmed Seid and Solomon Gizaw 1Department of Animal Science, Debark University, P.O. Box: 90, Debark, Ethiopia 2Jimma University, College of Agriculture and Veterinary Medicine, P.O. Box: 307, Jimma, Ethiopia 3International Livestock Research Institute (ILRI), P.O. Box: 5689, Addis Ababa, Ethiopia Abstract: The objectives of this study were to describe sheep and goats production systems comparatively in three districts of Oromia zone (ArtumaFursi, Dewachefa and JileTimuga). The study was performed based on household survey and field measurements. About 162 households (54 from each district) were involved. Data collected through questionnaire (survey) were statistically analyzed using SPSS. In the study area, the overall average number of sheep and goats per household were 7.19±4.34 and 11.90±6.70, respectively. Agro-pastoral (84.6%) and pastoral (15.4%) were the main production system in the study area. The primary reason of keeping sheep and goats in all districts was for cash income. Goat milk is consumed by respondents particularly in Artuma Fursi and Jile Timuga districts with index value of 0.019 and 0.078, respectively. On the other hand, all respondents in the study area reported that using sheep milk for home consumption is forbidden by their culture. Natural pasture and river water were the major sources of sheep and goats feed and water in both dry and wet seasons in the three districts.