CAID Services That Require Prior Auth List 10-01-2018.Xlsx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Colorectal Cancer Screening
CLINICAL MEDICAL POLICY Policy Name: Colorectal Cancer Screening Policy Number: MP-059-MD-PA Responsible Department(s): Medical Management Provider Notice Date: 03/19/2021 Issue Date: 03/19/2021 Effective Date: 04/19/2021 Next Annual Review: 02/2022 Revision Date: 02/17/2021 Products: Gateway Health℠ Medicaid Application: All participating hospitals and providers Page Number(s): 1 of 10 DISCLAIMER Gateway Health℠ (Gateway) medical policy is intended to serve only as a general reference resource regarding coverage for the services described. This policy does not constitute medical advice and is not intended to govern or otherwise influence medical decisions. POLICY STATEMENT Gateway Health℠ may provide coverage under the medical-surgical benefits of the Company’s Medicaid products for medically necessary colorectal cancer screening procedures. This policy is designed to address medical necessity guidelines that are appropriate for the majority of individuals with a particular disease, illness or condition. Each person’s unique clinical circumstances warrant individual consideration, based upon review of applicable medical records. (Current applicable Pennsylvania HealthChoices Agreement Section V. Program Requirements, B. Prior Authorization of Services, 1. General Prior Authorization Requirements.) Policy No. MP-059-MD-PA Page 1 of 10 DEFINITIONS Average-Risk Population – Patient population defined as having no personal history of adenomatous polyps, colorectal cancer, or inflammatory bowel disease (Crohn’s disease and Ulcerative Colitis); no family history of colorectal cancer or adenomatous polyps, familial adenomatous polyposis, or hereditary nonpolyposis colorectal cancer. High-Risk Population – Patient population defined as having a first-degree relative (sibling, parent, or child) who has had colorectal cancer or adenomatous polyps; OR family history of familial adenomatous polyposis; OR family history of hereditary non-polyposis colorectal cancer; OR family history of MYH- associated polyposis in siblings; OR diagnosis of Cowden syndrome. -

114.3 Cmr: Division of Health Care Finance and Policy Ambulatory Care
114.3 CMR: DIVISION OF HEALTH CARE FINANCE AND POLICY AMBULATORY CARE 114.3 CMR 40.00: RATES FOR SERVICES UNDER M.G.L. c. 152, WORKERS’ COMPENSATION ACT Section 40.01: General Provisions 40.02: General Definitions 40.03: Service and Rate Coverage Provisions 40.04: Provisions Affecting Eligible Providers 40.05: Policies for Individual Service Types 40.06: Fees 40.07: Appendices 40.08: Severability 40.01: General Provisions (1) Scope, Purpose and Effective Date. 114.3 CMR 40.00 governs the payment rates effective April 1, 2009 for purchasers of health care services under M.G.L. c. 152, the Workers’ Compensation Act. Payment rates for services provided by hospitals are set forth in 114.1 CMR 41.00. Program policies relating to medical necessity and clinical appropriateness are determined pursuant to M.G.L. c. 152 and 452 CMR 6.00. (2) Coverage. The payment rates set forth in 114.3 CMR 40.06 are full payment for services provided under M.G.L. c. 152, § 13, including any related administrative or overhead costs. The insurer, employer and health care service provider may agree upon a different payment rate for any service set forth in the fee schedule in 114.3 CMR 40.00. No employee may be held liable for the payment for health care services determined compensable under M.G.L. c. 152, § 13. (3) Administrative Bulletins. The Division may issue administrative bulletins to clarify substantive provisions of 114.3 CMR 40.00, or to publish procedure code updates and corrections. For coding updates and correction, the bulletin will list: (a) new code numbers for existing codes, with the corresponding cross references between existing and new codes numbers; (b) deleted codes for which there are no corresponding new codes; and (c) codes for entirely new services that require pricing. -

Geisinger Lewistown Hospital Published: March 25, 2019
Geisinger Lewistown Hospital Published: March 25, 2019 DESCRIPTION CHARGE Fine needle aspiration; without imaging guidance $ 607.00 Fine needle aspiration; without imaging guidance $ 286.00 Fine needle aspiration; with imaging guidance $ 2,218.00 Fine needle aspiration; with imaging guidance $ 1,691.00 Placement of soft tissue localization device(s) (eg, clip, metallic pellet, wire/needle, radioactive seeds), percutaneous, including imaging guidance; first lesion $ 1,979.00 Placement of soft tissue localization device(s) (eg, clip, metallic pellet, wire/needle, radioactive seeds), percutaneous, including imaging guidance; each $ 1,385.00 additional lesion (List separately in addition to code for primary procedure) Incision and drainage of abscess (eg, carbuncle, suppurative hidradenitis, cutaneous or subcutaneous abscess, cyst, furuncle, or paronychia); simple or single $ 657.00 Incision and drainage of abscess (eg, carbuncle, suppurative hidradenitis, cutaneous or subcutaneous abscess, cyst, furuncle, or paronychia); complicated or $ 986.00 multiple Incision and drainage of pilonidal cyst; simple $ 657.00 Incision and drainage of pilonidal cyst; complicated $ 3,726.00 Incision and removal of foreign body, subcutaneous tissues; simple $ 1,694.00 Incision and removal of foreign body, subcutaneous tissues; complicated $ 4,710.00 Incision and drainage of hematoma, seroma or fluid collection $ 3,470.00 Puncture aspiration of abscess, hematoma, bulla, or cyst $ 1,272.00 Puncture aspiration of abscess, hematoma, bulla, or cyst $ 657.00 Incision -

Confocal Laser Microscopy in Neurosurgery: State of the Art of Actual Clinical Applications
Journal of Clinical Medicine Review Confocal Laser Microscopy in Neurosurgery: State of the Art of Actual Clinical Applications Francesco Restelli 1, Bianca Pollo 2 , Ignazio Gaspare Vetrano 1 , Samuele Cabras 1, Morgan Broggi 1 , Marco Schiariti 1, Jacopo Falco 1, Camilla de Laurentis 1, Gabriella Raccuia 1, Paolo Ferroli 1 and Francesco Acerbi 1,* 1 Department of Neurosurgery, Fondazione IRCCS Istituto Neurologico Carlo Besta, 20133 Milan, Italy; [email protected] (F.R.); [email protected] (I.G.V.); [email protected] (S.C.); [email protected] (M.B.); [email protected] (M.S.); [email protected] (J.F.); [email protected] (C.d.L.); [email protected] (G.R.); [email protected] (P.F.) 2 Neuropathology Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, 20133 Milan, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-022-3932-309 Abstract: Achievement of complete resections is of utmost importance in brain tumor surgery, due to the established correlation among extent of resection and postoperative survival. Various tools have recently been included in current clinical practice aiming to more complete resections, such as neuronavigation and fluorescent-aided techniques, histopathological analysis still remains the gold-standard for diagnosis, with frozen section as the most used, rapid and precise intraoperative histopathological method that permits an intraoperative differential diagnosis. Unfortunately, due Citation: Restelli, F.; Pollo, B.; to the various limitations linked to this technique, it is still unsatisfactorily for obtaining real-time Vetrano, I.G.; Cabras, S.; Broggi, M.; intraoperative diagnosis. -

Diagnostic and Therapeutic Endoscopy of Biliary Diseases
DISEASES OF THE BILIARY TRACT, SERIES #6 Rad Agrawal, M.D., Series Editor Diagnostic and Therapeutic Endoscopy of Biliary Diseases by Yamini Subbiah, Shyam Thakkar, Elie Aoun The therapeutic approach to biliary diseases has undergone a paradigm shift over the past decade toward minimally invasive endoscopic interventions. This paper reviews the advances and different diagnostic and therapeutic endoscopic approaches to common biliary diseases including choledocholithiasis, benign and malignant biliary strictures and bile leaks. INTRODUCTION endoscopist to differentiate between benign and malig- ith the introduction of innovative endoscopic nant features, thus guiding decision making in real time. implements and options allowing for unprece- Wdented access to the biliary tree, the therapeutic COMMON BILE DUCT STONES approach to biliary diseases has undergone a significant Over 98% of biliary disorders are linked to gallstones. paradigm shift over the past decade toward minimally Stones are found in the common bile duct (CBD) in up invasive endoscopic interventions. The days where bil- to 18% of patients with symptomatic cholelithiasis (1). iary diseases were exclusively managed surgically are The vast majority of gallstones are cholesterol-rich, long gone, and much has changed since the first form in the gallbladder and gain access to the CBD via reported biliary sphincterotomies in 1974. The recent the cystic duct. De novo CBD stone formation is also developments in peroral cholangioscopy and new well described and is more common in patients of modalities of anchoring high resolution nasogastric Asian descent. These primary duct stones typically scopes in the bile duct offer the opportunity of direct have a higher bilirubin and a lower cholesterol content visualization of the bile duct lumen, which allows for and biliary stasis; further, bacterial infections have not only better identification of the underlying disease been implicated in their pathogenesis (2,3). -

Confocal Laser Endomicroscopy Platform Large Addressable Market: Early Cancer Diagnosis in GI, Urology, Interventional 2 Pulmonology, Others
Mauna Kea Technologies Corporate Presentation - November 2018 Disclaimer • This document has been prepared by Mauna Kea Technologies (the "Company") and is provided for information purposes only. • The information and opinions contained in this document speak only as of the date of this document and may be updated, supplemented, revised, verified or amended, and such information may be subject to significant changes. Mauna Kea Technologies is not under any obligation to update the information contained herein and any opinion expressed in this document is subject to change without prior notice. • The information contained in this document has not been independently verified. No representation, warranty or undertaking, express or implied, is made as to the accuracy, completeness or appropriateness of the information and opinions contained in this document. The Company, its subsidiary, its advisors and representatives accept no responsibility for and shall not be held liable for any loss or damage that may arise from the use of this document or the information or opinions contained herein. • This document contains information on the Company’s markets and competitive position, and more specifically, on the size of its markets. This information has been drawn from various sources or from the Company’s own estimates. Investors should not base their investment decision on this information. • This document contains certain forward-looking statements. These statements are not guarantees of the Company's future performance. These forward- looking statements relate to the Company's future prospects, developments and marketing strategy and are based on analyses of earnings forecasts and estimates of amounts not yet determinable. Forward-looking statements are subject to a variety of risks and uncertainties as they relate to future events and are dependent on circumstances that may or may not materialize in the future. -
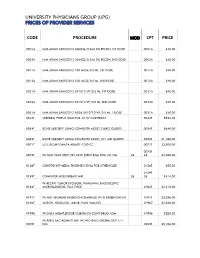
Code Procedure Cpt Price University Physicians Group
UNIVERSITY PHYSICIANS GROUP (UPG) PRICES OF PROVIDER SERVICES CODE PROCEDURE MOD CPT PRICE 0001A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 1ST DOSE 0001A $40.00 0002A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 2ND DOSE 0002A $40.00 0011A IMM ADMN SARSCOV2 100 MCG/0.5 ML 1ST DOSE 0011A $40.00 0012A IMM ADMN SARSCOV2 100 MCG/0.5 ML 2ND DOSE 0012A $40.00 0021A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 1ST DOSE 0021A $40.00 0022A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 2ND DOSE 0022A $40.00 0031A IMM ADMN SARSCOV2 AD26 5X10^10 VP/0.5 ML 1 DOSE 0031A $40.00 0042T CEREBRAL PERFUS ANALYSIS, CT W/CONTRAST 0042T $954.00 0054T BONE SURGERY USING COMPUTER ASSIST, FLURO GUIDED 0054T $640.00 0055T BONE SURGERY USING COMPUTER ASSIST, CT/ MRI GUIDED 0055T $1,188.00 0071T U/S LEIOMYOMATA ABLATE <200 CC 0071T $2,500.00 0075T 0075T PR TCAT PLMT XTRC VRT CRTD STENT RS&I PRQ 1ST VSL 26 26 $2,208.00 0126T CAROTID INT-MEDIA THICKNESS EVAL FOR ATHERSCLER 0126T $55.00 0159T 0159T COMPUTER AIDED BREAST MRI 26 26 $314.00 PR RECTAL TUMOR EXCISION, TRANSANAL ENDOSCOPIC 0184T MICROSURGICAL, FULL THICK 0184T $2,315.00 0191T PR ANT SEGMENT INSERTION DRAINAGE W/O RESERVOIR INT 0191T $2,396.00 01967 ANESTH, NEURAXIAL LABOR, PLAN VAG DEL 01967 $2,500.00 01996 PR DAILY MGMT,EPIDUR/SUBARACH CONT DRUG ADM 01996 $285.00 PR PERQ SAC AGMNTJ UNI W/WO BALO/MCHNL DEV 1/> 0200T NDL 0200T $5,106.00 PR PERQ SAC AGMNTJ BI W/WO BALO/MCHNL DEV 2/> 0201T NDLS 0201T $9,446.00 PR INJECT PLATELET RICH PLASMA W/IMG 0232T HARVEST/PREPARATOIN 0232T $1,509.00 0234T PR TRANSLUMINAL PERIPHERAL ATHERECTOMY, RENAL -

Orthopaedics AMPUTATION PRE-APPROVAL CODE DESCRIPTION REQUIRED PAYMENT INDICATORS PAYMENT RULES
Schedule of Benefits for Professional Fees 2020 Orthopaedics AMPUTATION PRE-APPROVAL CODE DESCRIPTION REQUIRED PAYMENT INDICATORS PAYMENT RULES Amputation, finger or thumb, primary or secondary, any joint or phalanx, single, including 3140 neurectomies; with direct closure (use also for traumatic amputations) No 3145 Amputation of two or more fingers No 3280 Amputation through forearm No 3415 Amputation through arm No 3464 Fore quarter amputation No 3645 Above knee amputation No 3690 Hind quarter amputation No 3790 Below knee amputation No 4255 Trans metatarsal amputation of foot No 4260 Trans metatarsal amputation of one toe No 4261 Trans metatarsal amputation of two or more toes No 4330 Trimming of stump following amputation of limb No ANKLE PRE-APPROVAL CODE DESCRIPTION REQUIRED PAYMENT INDICATORS PAYMENT RULES 3955 Arthrodesis of ankle joint No Arthroscopy, ankle, with or without removal of loose body or foreign body, with or without 3956 synovectomy, debridement (I.P.) No Independent Procedure, Day Care Arthroscopy, ankle, surgical, excision of osteochondral defect of talus and/ or tibia, including 3961 drilling of the defect (I.P.) No Independent Procedure 1 Night Only Arthroscopically aided repair of large osteochondritis dissecans lesion, talar dome fracture, or 3962 tibial plafond fracture, with or without internal fixation (includes arthroscopy) (I.P.) No Independent Procedure 3963 Arthroscopy, subtalar joint, surgical, with subtalar arthrodesis (I.P.) No Independent Procedure 3965 Fracture of medial or lateral malleolus (1st -

Talectomy (Astragalectomy) and Tibiocalcaneal Arthrodesis Following Traumatic Talus Fracture-Dislocation
Talectomy (astragalectomy) and tibiocalcaneal arthrodesis following traumatic talus fracture-dislocation The Foot and Ankle Online Journal 12 (2): 4 1 by Dr Alison Zander, MBBCh, BSc (hons), MSc (PHNutr) , Mr Anirudh Gadgil, MBBS, M.S. (Orth), 2 3* FRCS (Ed), FRCS (Trauma & Ortho) , Derek Protheroe, BSc(Hons), MSc, PgDip Talus fractures occur rarely but are often associated with complications and functional limitations. Urgent reduction of associated dislocations is recommended with open-reduction and internal fixation of displaced fractures when adjacent soft tissue injury permits [1]. However, it is important to remember that there is a high incidence of long term complications, along with a significant impact on activities of daily living and quality of life. This case report describes the successful treatment of a severely comminuted talar fracture dislocation with primary talectomy and tibio-calcaneal arthrodesis. A reminder that in selected cases that the talectomy (astragalectomy) may be a viable alternative. Keywords: talus, comminuted, tibiocalcaneal arthrodesis, fusion, talectomy, astragalectomy, trauma, avascular-necrosis, AVN This is an Open Access article distributed under the terms of the Creative Commons Attribution License. It permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ©The Foot and Ankle Online Journal (www.faoj.org), 2019. All rights reserved. alus fractures account for less than 1% of all surfaces is precluded secondary to comminution [4]. fractures, they may be caused by high-energy The talus is the second largest of the tarsal bones, trauma, and any other form of forced with more than half of its surface being covered with dorsiflexion injury to the ankle and foot [1]. -

Cycloid Scanning for Wide Field Optical Coherence Tomography Endomicroscopy and Angiography in Vivo
Cycloid scanning for wide field optical coherence tomography endomicroscopy and angiography in vivo The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Liang, Kaicheng et al. "Cycloid scanning for wide field optical coherence tomography endomicroscopy and angiography in vivo." Optica 5, 1 (January 2018): 36-43 © 2018 Optical Society of America As Published http://dx.doi.org/10.1364/OPTICA.5.000036 Publisher OSA Publishing Version Author's final manuscript Citable link https://hdl.handle.net/1721.1/121433 Terms of Use Creative Commons Attribution-Noncommercial-Share Alike Detailed Terms http://creativecommons.org/licenses/by-nc-sa/4.0/ HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Optica. Manuscript Author Author manuscript; Manuscript Author available in PMC 2018 April 20. Published in final edited form as: Optica. 2018 January 20; 5(1): 36–43. doi:10.1364/OPTICA.5.000036. Cycloid scanning for wide field optical coherence tomography endomicroscopy and angiography in vivo Kaicheng Liang1,†, Zhao Wang1,†, Osman O. Ahsen1, Hsiang-Chieh Lee1, Benjamin M. Potsaid1,2, Vijaysekhar Jayaraman3, Alex Cable2, Hiroshi Mashimo4,5, Xingde Li6, and James G. Fujimoto1,* 1Department of Electrical Engineering and Computer Science, Research Laboratory of Electronics, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA 2Thorlabs, Newton, New Jersey 07860, USA 3Praevium Research, Santa Barbara, California 93111, USA 4Veterans Affairs Boston Healthcare System, Boston, Massachusetts 02130, USA 5Harvard Medical School, Boston, Massachusetts 02115, USA 6Department of Biomedical Engineering, Johns Hopkins University, Baltimore, Maryland 21218, USA Abstract Devices that perform wide field-of-view (FOV) precision optical scanning are important for endoscopic assessment and diagnosis of luminal organ disease such as in gastroenterology. -

Fees -As of 6 12 2019.Xls
• Timed charges: Some charges such as anesthesia are based on a units of time, so the charges may vary based on the units charged. • Drugs and implants: Drugs, implants and supplies are priced individually based on the cost loaded into our information systems at the time of charging, so they do not have individual prices listed. The typical methodology will take the cost in place at the time, multiplied by a markup percentage. • Estimated Total Charges of Procedure/Stays/Visits etc can be obtained by contacting -AJ Karpinski at 218-546-2507. All charges are then subject to Insurance contract payer reductions. To get any accurate representation of patient final cost, I would strongly encourage contacting us to accurately predict final price -costs of services State Mandated Clinic Price Transparency – Average. Charge Medicare Medicaid Cuyuna Regional Medical Center - Clinic Commecial Reim. Office/outpatient visit new Level 1 $190 $76 $45 $34 Office/outpatient visit new Level 2 $396 $130 $75 $57 Office/outpatient visit new Level 3 $574 $186 $107 $82 Office/outpatient visit new Level 4 $708 $283 $163 $126 Office/outpatient visit est Level 1 $96 $36 $22 $16 Office/outpatient visit est Level 2 $187 $76 $44 $34 Office/outpatient visit est Level 3 $424 $127 $73 $56 Office/outpatient visit est Level 4 $549 $187 $108 $83 Office/outpatient visit est Level 5 $624 $245 $145 $112 Per pm reeval est pat infant $300 $176 Non-cov. $77 Prev visit est age 1-4 $347 $184 Non-cov. $82 Prev visit est age 18-39 $432 $205 Non-cov. -

TIBIOTALOCALCANEAL and PANTALAR ARTHRODESIS Foot
TIBIOTALOCALCANEAL AND PANTALAR ARTHRODESIS Foot and Ankle Clinics Author: George E. Quill, Jr., M.D. Since the report by Albert in 1882, arthrodesis has been an accepted treatment for painful arthritis of the ankle and subtalar joints.3 For the purpose of this and other orthopaedic articles, surgical fusion of the ankle (tibiotalar) and subtalar (talocalcaneal) joints at the same operative sitting will be termed tibiotalocalcaneal arthrodesis. Fusion of the talus to all the bones articulating with it (distal tibia, calcaneus, tarsonavicular, and cuboid) is termed pantalar arthrodesis.13,20 Numerous techniques exist describing fusion approaches and implants, all having in common a similar goal: a solid, pain free arthrodesis in a biomechanically stable and functional position.1-31 The indications for tibiotalocalcaneal arthrodesis include avascular necrosis of the talus or a failed total ankle arthroplasty with subtalar intrusion.14,16,20,21 The failed ankle fusion with insufficient talar body, as well as patients with rheumatoid or osteoarthritic involvement of these joints are also candidates for tibiotalocalcaneal fusion.8,16,23,25,30 Other indications include the sequelae of trauma and the severe deformity of untreated clubfoot and neuromuscular disease.5,7,20,23,24 Patients with Charcot arthropathy, skeletal defects after tumor reconstruction, pseudarthrosis, as well as fixed and various flail deformities about the hindfoot and ankle are also candidates for tibiotalocalcaneal and, in certain instances, pantalar arthrodesis.4,9,18,20,22,27,28