Lepidoptera: Pterophoridae) with Description of Four New Species Deborah L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A European Species for the Biological Control of Invasive Swallow-Worts (Vincetoxicum Spp.) in North America
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Department of Agriculture: Agricultural Publications from USDA-ARS / UNL Faculty Research Service, Lincoln, Nebraska 1-8-2014 HYPENA OPULENTA (EREBIDAE): A EUROPEAN SPECIES FOR THE BIOLOGICAL CONTROL OF INVASIVE SWALLOW-WORTS (VINCETOXICUM SPP.) IN NORTH AMERICA Jim Young United States of America Department of Agriculture, [email protected] Aaron S. Weed Dartmouth College, Hanover, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/usdaarsfacpub Young, Jim and Weed, Aaron S., "HYPENA OPULENTA (EREBIDAE): A EUROPEAN SPECIES FOR THE BIOLOGICAL CONTROL OF INVASIVE SWALLOW-WORTS (VINCETOXICUM SPP.) IN NORTH AMERICA" (2014). Publications from USDA-ARS / UNL Faculty. 2339. https://digitalcommons.unl.edu/usdaarsfacpub/2339 This Article is brought to you for free and open access by the U.S. Department of Agriculture: Agricultural Research Service, Lincoln, Nebraska at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Publications from USDA-ARS / UNL Faculty by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 162 162 JOURNAL OF THE LEPIDOPTERISTS ’ S OCIETY Journal of the Lepidopterists’ Society 68(3), 2014, 162 –166 HYPENA OPULENTA (EREBIDAE): A EUROPEAN SPECIES FOR THE BIOLOGICAL CONTROL OF INVASIVE SWALLOW-WORTS ( VINCETOXICUM SPP.) IN NORTH AMERICA JIM YOUNG , P HD. United States of America Department of Agriculture, Animal and Plant Health Inspection Service, Plant Protection and Quarantine. 2400 Broening Hwy. Ste 102, Baltimore, MD 21124. [email protected] AND AARON S. W EED Department of Biological Sciences, Dartmouth College, Hanover, NH 03755, [email protected] ABSTRACT. -

Entomology 101 Jason J
Entomology 101 Jason J. Dombroskie Manager, Cornell U. Insect Collection Coordinator, Insect Diagnostic Lab This material [email protected] can only be used for CCE MGV audiences. Outline • What is an insect? • Anatomy • Life cycles • Diversity • Major orders • Herbivory Corydalus cornutus Cornell U. Insect Collection • > 7 million specimens • ~200 000 species • worldwide coverage • http://cuic.entomology.cornell.edu/ • on facebook Insect Diagnostic Lab • ~700 IDs per year • 10-20 000 IDs for NYS Dept. Ag. & Markets • occasionally IDs can be made from a photo • mostly local, but some submissions worldwide • $25 fee • http://entomology.cornell.edu/IDL Arthropods Regier, et al. 2005 What is an insect? • 3 main body parts • 6 jointed legs • 1 pair of antennae • compound eyes • usually some sort of metamorphosis Booneacris glacialis Head • antennae • mouthparts • compound eyes • ocelli Monochamus scutellatus Popillia japonica Tetanocera sp. Antheraea polyphemus wikimedia commons labrum maxilla mandible labium Corydalus cornutus Polygonia progne Aedes sp. Hybomitra zonalis Monochamus notatus Aeshna canadensis Isoptera Darapsa myron Thorax • six legs • four wings or less • muscular Amateur Entomologists’ Society Entomologists’ Amateur Limenitis archippus Lethocerus americanus Zeugomantispa minuta Machimus sp. with Herpetogramma pertextalis wikimedia commons Tipula apicalis Cybister fimbriolatus Elasmucha lateralis Automeris io Abdomen • internal organs • genitalia • ovipositor Ophiogomphus rupinsulensis Lauxania shewelli Merope tuber Adoxophyes -

SYSTEMATICS of the MEGADIVERSE SUPERFAMILY GELECHIOIDEA (INSECTA: LEPIDOPTEA) DISSERTATION Presented in Partial Fulfillment of T
SYSTEMATICS OF THE MEGADIVERSE SUPERFAMILY GELECHIOIDEA (INSECTA: LEPIDOPTEA) DISSERTATION Presented in Partial Fulfillment of the Requirements for The Degree of Doctor of Philosophy in the Graduate School of The Ohio State University By Sibyl Rae Bucheli, M.S. ***** The Ohio State University 2005 Dissertation Committee: Approved by Dr. John W. Wenzel, Advisor Dr. Daniel Herms Dr. Hans Klompen _________________________________ Dr. Steven C. Passoa Advisor Graduate Program in Entomology ABSTRACT The phylogenetics, systematics, taxonomy, and biology of Gelechioidea (Insecta: Lepidoptera) are investigated. This superfamily is probably the second largest in all of Lepidoptera, and it remains one of the least well known. Taxonomy of Gelechioidea has been unstable historically, and definitions vary at the family and subfamily levels. In Chapters Two and Three, I review the taxonomy of Gelechioidea and characters that have been important, with attention to what characters or terms were used by different authors. I revise the coding of characters that are already in the literature, and provide new data as well. Chapter Four provides the first phylogenetic analysis of Gelechioidea to include molecular data. I combine novel DNA sequence data from Cytochrome oxidase I and II with morphological matrices for exemplar species. The results challenge current concepts of Gelechioidea, suggesting that traditional morphological characters that have united taxa may not be homologous structures and are in need of further investigation. Resolution of this problem will require more detailed analysis and more thorough characterization of certain lineages. To begin this task, I conduct in Chapter Five an in- depth study of morphological evolution, host-plant selection, and geographical distribution of a medium-sized genus Depressaria Haworth (Depressariinae), larvae of ii which generally feed on plants in the families Asteraceae and Apiaceae. -
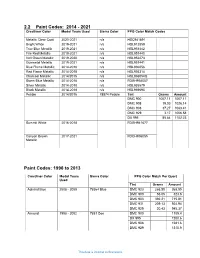
Paint Codes: 2014 - 2021 Crestliner Color Model Years Used Sierra Color PPG Color Match Codes
2.2 Paint Codes: 2014 - 2021 Crestliner Color Model Years Used Sierra Color PPG Color Match Codes Metallic Clear Coat 2020-2021 n/a HSC941891 Bright White 2019-2021 n/a HSL912859 True Blue Metallic 2019-2021 n/a HSL951442 Fire Red Metallic 2019-2021 n/a HSL951440 Volt Green Metallic 2019-2020 n/a HSL952273 Gunmetal Metallic 2019-2021 n/a HSL951441 Blue Flame Metallic 2014-2018 n/a HSL936056 Red Flame Metallic 2014-2018 n/a HSL936314 Charcoal Metallic 2014/2015 n/a HSL936054Q Storm Blue Metallic 2014-2016 n/a FDGH936057 Silver Metallic 2014-2018 n/a HSL935579 Black Metallic 2014-2018 n/a HSL939695 Pebble 2014/2015 78874 Pebble Tint Grams Amount DMC 900 1007.11 1007.11 DMC 908 19.03 1026.14 DMC 903 37.27 1063.41 DMC 929 3.17 1066.58 DX 995 85.64 1152.23 Summit White 2016-2018 FDGH941677 Canyon Brown 2017-2021 FDGH936055 Metallic Paint Codes: 1996 to 2013 Crestliner Color Model Years Sierra Color PPG Color Match Per Quart Used Tint Grams Amount Admiral Blue 2008 - 2009 78364 Blue DMC 923 268.55 268.55 DMC 900 55.05 323.6 DMC 903 392.21 715.81 DMC 931 209.13 924.94 DMC 929 20.43 945.37 Almond 1998 - 2002 7881 Doe DMC 900 1105.4 DX 995 1200.6 DMC 908 1281.6 DMC 929 1315.9 This data is internal to Brunswick. DMC 923 1325 Aluminum 2002 - 2003 7890 Gray Metallic Low DMC 981 800.61 800.61 Gloss DMC 983 103.94 904.55 Ash Gray 1999 7864 Ranger Light Gray DMC 900 830.1 DMC 984 1015.6 DMC 902 1143.8 DX 995 1234.5 DMC 923 1242.3 Baroque Burgundy 1999 7822 Burgundy 1999 DMC 933 641.4 DX 995 762.1 DMC 900 870.9 DMC 931 966.5 DMC 929 1044.9 DMC 901 1089.3 -

Welcome to the Skagway Historic District Color Palette
Skagway Historic District Architectural Paint Color Palette INTRODUCTION The 80 paint colors listed here are those that may be used on the exterior of buildings in the Skagway Historic District. Unless you have good historical evidence that your building was painted otherwise, you must choose from the colors shown here. Many of the colors in this palette have been identified by laboratory analysis of actual paint samples collected from Skagway’s historic buildings and can be seen on buildings that have been restored by the National Park Service. The remaining colors are those that were offered by U.S. paint manufacturers of the 1897‐1910 period and were likely available in Skagway. Period Aesthetics and Paint Schemes Generally, late Victorian paint schemes (i.e.: c.1870‒1900) were characterized by earth colors and greens, often in surprisingly dark shades. Whites and creams were not in fashion. Closely related body and trim colors were often favored. (An example of this may be seen on the Mascot Saloon building which has been restored by the NPS to its 1898 appearance.) Sash colors were usually dark green, black, or occasionally, red. (Sash refers to the wooden parts of a window that surround the glass. It does not include the trim surrounding the window such as the window frame and sill.) High Victorian style buildings, (The Pack Train building, the Golden North Hotel building, and the Mulvihill house in Skagway are examples of this style), having multiple stories and complicated patterns and structures such as scalloped shingles, multiple beltcourses, bracketed cornices, towers, etc., were often painted with two or three body colors as well as two trim colors, a sash color, and sometimes even an accent color. -

Wildlife Review Cover Image: Hedgehog by Keith Kirk
Dumfries & Galloway Wildlife Review Cover Image: Hedgehog by Keith Kirk. Keith is a former Dumfries & Galloway Council ranger and now helps to run Nocturnal Wildlife Tours based in Castle Douglas. The tours use a specially prepared night tours vehicle, complete with external mounted thermal camera and internal viewing screens. Each participant also has their own state- of-the-art thermal imaging device to use for the duration of the tour. This allows participants to detect animals as small as rabbits at up to 300 metres away or get close enough to see Badgers and Roe Deer going about their nightly routine without them knowing you’re there. For further information visit www.wildlifetours.co.uk email [email protected] or telephone 07483 131791 Contributing photographers p2 Small White butterfly © Ian Findlay, p4 Colvend coast ©Mark Pollitt, p5 Bittersweet © northeastwildlife.co.uk, Wildflower grassland ©Mark Pollitt, p6 Oblong Woodsia planting © National Trust for Scotland, Oblong Woodsia © Chris Miles, p8 Birdwatching © castigatio/Shutterstock, p9 Hedgehog in grass © northeastwildlife.co.uk, Hedgehog in leaves © Mark Bridger/Shutterstock, Hedgehog dropping © northeastwildlife.co.uk, p10 Cetacean watch at Mull of Galloway © DGERC, p11 Common Carder Bee © Bob Fitzsimmons, p12 Black Grouse confrontation © Sergey Uryadnikov/Shutterstock, p13 Black Grouse male ©Sergey Uryadnikov/Shutterstock, Female Black Grouse in flight © northeastwildlife.co.uk, Common Pipistrelle bat © Steven Farhall/ Shutterstock, p14 White Ermine © Mark Pollitt, -

Members' Day Sale
Name Description This Mexican species is called the “Octopus Agave” because of its beautifully twisting and arching leaves. A real showstopper in the landscape, it grows slowly to 4’ tall. After about ten years, it produces a magnificent inflorescence of bright yellow flowers. It will thrive in full sun with no supplemental irrigation or soil amendments. Be sure that the Agave vilmoriniana planting site drains well, and give it a small application of low nitrogen granular fertilizer before the onset of the summer rainy season. Dwarf Elephant Ear has large leaves and tends to form a clump. It is one of the easiest alocasias to grow in the garden. It can take sun to shade, though prefers brighter light. It Alocasia gageana responds well to regular watering, but is also tolerant of neglect. This is a dramatically variegated Alocasia selection with yellow‐white veins and spots against a dark green background of the heart‐shaped leaves. It is fast growing and prefers Alocasia sp. bright but indirect light and can probably take full sun as well. Good drainage and irrigation are important as is fairly heavy fertilization to make these plants look their best. An excellent landscape plant, this Caribbean Anthurium has glossy heart‐shaped leaves held in a rosette. It is quite forgiving of neglect and well adapted to South florida Anthurium cf. cordifolium preferring part shade and good drainage. A climbing Anthurium that is well‐adapted to South Florida. It does best in part‐shade to shade and produces distinctive palmately divided leaves. It also performs well as a Anthurium digitatum houseplant. -

Plume Moths of Family Pterophoridae (Microlepidoptera) from Shiwaliks of North-West India
Rec. zool. Surv. India: Vol. 119(3)/ 256-262, 2019 ISSN (Online) : 2581-8686 DOI: 10.26515/rzsi/v119/i3/2019/143334 ISSN (Print) : 0375-1511 Plume moths of family Pterophoridae (Microlepidoptera) from Shiwaliks of North-West India H. S. Pooni1*, P. C. Pathania2 and Amit Katewa1 1Department of Zoology and Environmental Sciences, Punjabi University, Patiala - 1470002, Punjab, India; [email protected] 2Zoological Survey of India, M-Block, New Alipore, Kolkata - 700 053, West Bengal, India Abstract Survey tours were undertaken for the collection of Pterophorid moths from various localities falling in the jurisdiction of North-Western Shiwaliks. In all, 26 species belonged to 18 genera of the family Pterophoridae(25 species of subfamily and remarks for all the species are also provided in detail. Pterophorinae and 01 Deuterocopinae) were examined and identified. The keys to subfamilies, synonymy, distribution Keywords: Microlepidoptera, North-West, Plume Moths, Pterophoridae Introduction of these moths, the taxonomical study is very difficult and the same moths group poses very serious problems The Microlepidoptera is one of the large groups of in field collections, pinning, stretching, labelling and as moths under order Lepidoptera. On world basis, 45735 well as in identification. Keeping in mind all above, the species belonging to 4626 genera of 73 families under 19 present research is undertaken on the Pterophorid moths superfamilies are present. The superfamily Pterophoroidea from the area under reference. is a unique group from other Lepidopteran insects is having slender moths, long and slender legs and long Material and Methods abdomen and wings narrow clefted. The wings are narrow. -

PLUME MOTHS of AFGHANISTAN (LEPIDOPTERA, PTEROPHORIDAE) 1Altai State University, Lenina 61
Biological Bulletin of Bogdan Chmelnitskiy Melitopol State Pedagogical University 183 UDC 595.7(262.81) Peter Ustjuzhanin,1,6* Vasily Kovtunovich,2 Igor Pljushtch,3 Juriy Skrylnik4, Oleg Pak5 PLUME MOTHS OF AFGHANISTAN (LEPIDOPTERA, PTEROPHORIDAE) 1Altai State University, Lenina 61. RF-656049. Barnaul, Russia. 2 Moscow Society of Nature Explorers. Home address: Russia, Moscow, 121433, Malaya Filevskaya str., 24/1, app. 20. 3Schmalhausen Institute of Zoology, National Academy of Science of Ukraine, Bogdan Khmielnitski str., 15, 01601, Kiev, Ukraine. 4 Ukrainian Research Institute of Forestry & Forest Melioration, 61024, Pushkinska str. 86, Kharkov, Ukraine. 5 Donetsk National University, Faculty of Biology, Shchors str., 46, 83050, Donetsk, Ukraine. 6*Corresponding author. E-mail: [email protected] New data on Pterophoridae from Afghanistan are considered. A checklist of Pterophoridae species of the fauna of Afghanistan is presented, as including 32 species of 14 genera. Merrifieldia tridactyla is for the first time recorded for the fauna of Afghanistan. The basic literature on the Afghanistan Pterophoridae were used in the study. Key words: Pterophoridae, plume moths, Afghanistan, fauna, new data. INTRODUCTION Many Pterophoridae as Cossidae are specific inhabitants of the arid regions of the Palaearctic. Usually deserts are good zoogeographical barriers preventing from mixing the faunas of different zoogeographical regions (Yakovlev & Dubatolov 2013; Yakovlev, 2015; Yakovlev et al., 2015). Until now there were no special publications on Pterophoridae from Afghanistan. The first description of a new species of Pterophoridae, Stenoptilia nurolhaki, from Afghanistan was in the work by Amsel (1967), In a series of works by Ernst Arenberger (1981, 1987, 1995), six new species were described from Afghanistan. -

A New Species of Oidaematophorus (Lepidoptera: Pterophoridae) from Chingaza National Natural Park in Colombia
TENNENT ET AL.: New species of Epimastidia and Paraduba HERNÁNDEZ ET AL.: New species of Oidaematophorus TROP. LEPID. RES., 24(1): 15-21, 2014 15 A NEW SPECIES OF OIDAEMATOPHORUS (LEPIDOPTERA: PTEROPHORIDAE) FROM CHINGAZA NATIONAL NATURAL PARK IN COLOMBIA Linda C. Hernández1, Luz Stella Fuentes2a, Gonzalo E. Fajardo2b, and Deborah L. Matthews3 1Departamento de Entomología, Centro de Bio-Sistemas Universidad Jorge Tadeo Lozano., Bogotá, Colombia, [email protected]; 2Facultad de Ciencias Biológicas e Ingeniería, Universidad Jorge Tadeo Lozano., Bogotá, Colombia, [email protected], [email protected]; 3McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, [email protected] Abstract - Oidaematophorus espeletiae, sp. nov., is described from the Chingaza páramo in Colombia. The life history, external characters of the adult, male and female genitalia, final instar larva, and pupa are described and illustrated. This moth species is widely distributed in the páramo. Larvae cause damage in meristem leaves of frailejones (Espeletia spp., Asteraceae). Identification and continuing studies of this moth are important to determine its potential role in the reported death of numerous frailejones in the area. The hosts, Espeletia grandiflora and E. uribei are some of the keystone species of the páramo ecosytem. Resumen - Se describe Oidaematophorus espeletiae, sp. nov del páramo de Chingaza, Colombia. Se describen e ilustran la historia de vida, caracteres externos del adulto, genitalia de macho y hembra, último instar larval, y pupa. Esta polilla se encuentra ampliamente distribuida en el páramo. Las larvas ocasionan daños en las hojas del meristemo de los frailejones (Espeletia spp., Asteraceae). -

Moths of the Douglas Lake Region (Emmet and Cheboygan Counties), Michigan: VI
The Great Lakes Entomologist Volume 35 Number 1 - Spring/Summer 2002 Number 1 - Article 10 Spring/Summer 2002 April 2002 Moths of the Douglas Lake Region (Emmet and Cheboygan Counties), Michigan: VI. Miscellaneous Small Families (Lepidoptera) Edward G. Voss University of Michigan Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Voss, Edward G. 2002. "Moths of the Douglas Lake Region (Emmet and Cheboygan Counties), Michigan: VI. Miscellaneous Small Families (Lepidoptera)," The Great Lakes Entomologist, vol 35 (1) Available at: https://scholar.valpo.edu/tgle/vol35/iss1/10 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Voss: Moths of the Douglas Lake Region (Emmet and Cheboygan Counties), 2002 THE GREAT LAKES ENTOMOLOGIST 53 MOTHS OF THE DOUGLAS LAKE REGION (EMMET AND CHEBOYGAN COUNTIES), MICHIGAN: VI. MISCELLANEOUS SMALL FAMILIES (LEPIDOPTERA) Edward G. Voss1 ABSTRACT Forty-seven species in nine families of Lepidoptera (Hepialidae, Psychidae, Alucitidae, Sesiidae, Cossidae, Limacodidae, Thyrididae, Pterophoridae, Epiplemi- dae) are listed with earliest and latest recorded flight dates in Emmet and Cheboy- gan counties, which share the northern tip of the Lower Peninsula of Michigan. The records are from the principal institutional and private collections of Michigan moths and continue the documented listing of Lepidoptera in the region. ____________________ Emmet and Cheboygan counties share the northern tip of the Lower Peninsula of Michigan, the former bordered on the west by Lake Michigan and the latter, on the east by Lake Huron. -

Adaina Primulacea Meyrick, 1929: a Gall-Inducing Plume Moth of Siam Weed from South Florida and the Neotropics (Lepidoptera: Pterophoridae)
64 TROP. LEPID. RES., 19(2):64-70, 2009 MATTHEWS & MAHARAJH: Life history of Adaina plume moth ADAINA PRIMULACEA MEYRICK, 1929: A GALL-INDUCING PLUME MOTH OF SIAM WEED FROM SOUTH FLORIDA AND THE NEOTROPICS (LEPIDOPTERA: PTEROPHORIDAE) Deborah L. Matthews1 and Boudanath V. Maharajh2, † 1McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, P. O. Box 112710, Gainesville, Florida 32611-2710, USA; 2Department of Entomology and Nematology, Fort Lauderdale Research and Education Center, University of Florida, Institute of Food and Agricultural Sciences, 3205 College Avenue, Fort Lauderdale, Florida 33314, USA. † Deceased 22 August 2009 Abstract- The life history of Adaina primulacea Meyrick is described and illustrated. Larvae induce formation of stem galls on Siam Weed, Chromolaena odorata (L.) R.M.King & H. Rob., and feed and pupate within these galls. This neotropical species was discovered in South Florida in 1993 and has since been exported for biological control studies. The identity of the species is established in this paper by comparison of reared specimens with images of the holotype from Panama. The female genitalia are described and illustrated for the first time. Key Words: cecidogenous, Chromolaena odorata, Eupatorium cannabinum, Siam Weed, Asteraceae, stem galls, Adaina primulacea, A. microdactyla, A. simplicius, A. bipunctata, biological control, larvae, pupae, Pterophoroidea, Pterophorinae The genus Adaina Tutt, 1905 includes 28 species worldwide and competes with crops as well as native vegetation in (Gielis 2003), 20 of which occur in the Neotropical Region. enviromentally sensitive areas. It is a fire hazard in grasslands The type species, Adaina microdactyla (Hübner) is widespread and a problem weed in pastures as it is toxic to cattle.