Conservation Survey of the Tokelau
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Meadow Argus Junonia Villida Calybe
Butterfly GardeningFact sheet Nymphalidae family Meadow Argus Junonia villida calybe Abundance in Adelaide area: Common Flight: Throughout the year Wingspan: m 40 mm; f 43 mm Mature larva length: 32 mm The Meadow Argus is a common butterfly that occurs throughout the Adelaide region. Unfortunately, some of its caterpillar food plants are weeds and not desirable in the home garden. Keen gardeners could try some of the native caterpillar food plants listed here. Because its weedy food plants are so widespread and the butterfly is a strong flier that travels widely, even if you don’t succeed in establishing a breeding colony of Argus butterflies, you should still have them as regular visitors to your nectar plants. Caterpillar food plants: Native herbaceous How these eyespots work is a topic of dispute. plants and many invasive weeds. The caterpillars They either scare a predator into thinking the eat the soft parts of the plants. expanded wings of the butterflies are actually the head of a much larger animal, or attract the Adelaide native species: Common Purslane attention of an attacking bird or lizard, causing (Portulaca oleracea), Fairy Fan-flower (Scaevola them to target the edges of the wings, thus aemula). leaving the body and vital organs of the body of the insect alone. Other South Australian species: Spreading Nut-heads (Epaltes australis), Goodenia spp., The butterfly is a brown colour above, with a pair Fan-flowers (Scaevola spp.), Bluerod (Stemodia of blue centered, black eyespots, surrounded in florulenta). orange, near the outer margin of the wings. Foreign species: Snapdragons and weeds— Underneath, the forewing is quite similar to Centaury, Lippia*, Ribwort* (Plantago lanceolata), the upper surface, though much paler. -

The Place of Alcohol in the Lives of People from Tokelau, Fiji, Niue
The place of alcohol in the lives of people from Tokelau, Fiji, Niue, Tonga, Cook Islands and Samoa living in New Zealand: an overview The place of alcohol in the lives of people from Tokelau, Fiji, Niue, Tonga, Cook Islands and Samoa living in New Zealand: an overview A report prepared by Sector Analysis, Ministry of Health for the Alcohol Advisory Council of New Zealand ALAC Research Monograph Series: No 2 Wellington 1997 ISSN 1174-1856 ISBN 0-477-06317-9 Acknowledgments This particular chapter which is an overview of the reports from each of the six Pacific communities would not have been possible without all the field teams and participants who took part in the project. I would like to thank Ezra Jennings-Pedro, Terrisa Taupe, Tufaina Taupe Sofaia Kamakorewa, Maikali (Mike) Kilioni, Fane Malani, Tina McNicholas, Mere Samusamuvodre, Litimai Rasiga, Tevita Rasiga, Apisa Tuiqere, Ruve Tuivoavoa, Doreen Arapai, Dahlia Naepi, Slaven Naepi, Vili Nosa, Yvette Guttenbeil, Sione Liava’a, Wailangilala Tufui , Susana Tu’inukuafe, Anne Allan-Moetaua, Helen Kapi, Terongo Tekii, Tunumafono Ken Ah Kuoi, Tali Beaton, Myra McFarland, Carmel Peteru, Damas Potoi and their communities who supported them. Many people who have not been named offered comment and shared stories with us through informal discussion. Our families and friends were drawn in and though they did not formally participate they too gave their opinions and helped to shape the information gathered. Special thanks to all the participants and Jean Mitaera, Granby Siakimotu, Kili Jefferson, Dr Ian Prior, Henry Tuia, Lita Foliaki and Tupuola Malifa who reviewed the reports and asked pertinent questions. -
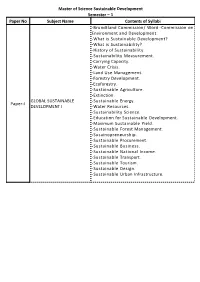
Master of Science Sustainable Development Semester – 1 Paper
Master of Science Sustainable Development Semester – 1 Paper No Subject Name Contents of Syllabi -Brundtland Commission/ Word -Commission on Environment and Development. -What is Sustainable Development? -What is Sustainability? -History of Sustainability. -Sustainability Measurement. -Carrying Capacity. -Water Crisis. -Land Use Management. -Forestry Development. -Ecoforestry. -Sustainable Agriculture. -Extinction. GLOBAL SUSTAINABLE -Sustainable Energy. Paper-I DEVELOPMENT I -Water Resources. -Sustainability Science. -Education for Sustainable Development. -Maximum Sustainable Yield. -Sustainable Forest Management. -Susainopreneurship. -Sustainable Procurement. -Sustainable Business. -Sustainable National Income. -Sustainable Transport. -Sustainable Tourism. -Sustainable Design. -Sustainable Urban Infrastructure. Master of Science Sustainable Development -What is Biodiversity? -Measurement of Biodiversity. -Species Richness. -Shannon Index. -2010 Biodiversity Indicators- Partnership. -Evolution. -Evolutionary Thought. -How does Evolution Occur? -Timeline of Evolution. BIODIVERSITY -Social Effect of Evolutionary Theory. Paper-II CONSERVATION & -Objections of Evolution. MANAGEMENT I -Population Genetics. -Genetics. -Heredity. -Mutation. -Molecular Evolution. -Genetic Recombination. -Sexual Reproduction. -Gene Flow. -Hybrid. -What is Energy? -History of Energy. -Timeline of Thermodynamics. -Units of Energy. -Potential Energy. -Elastic Energy. -Kinetic Energy. -Internal Energy. -Electromagnetism. -Electricity. GLOBAL ENERGY POLICIES Paper-III -

Tokelau the Last Colony?
Tokelau The last colony? TONY ANGELO (Taupulega) is, and long has been, the governing body. The chairman (Faipule) of the council and a village head ITUATED WELL NORTH OF NEW ZEALAND and (Pulenuku) are elected by universal suffrage in the village SWestern Samoa and close to the equator, the small every three years. The three councils send representatives atolls of Tokelau, with their combined population of about to form the General Fono which is the Tokelau national 1600 people, may well be the last colony of New Zealand. authority; it originally met only once or twice a year and Whether, when and in what way that colonial status of advised the New Zealand Government of Tokelau's Tokelau will end, is a mat- wishes. ter of considerable specula- The General Fono fre- lion. quently repeated advice, r - Kirlb•ll ·::- (Gifb•rr I•) The recently passed lbn•b'a ' ......... both to the New Zealand (Oc: ..n I} Tokelau Amendment Act . :_.. PMtnb 11 Government and to the UN 1996- it received the royal Committee on Decoloni • •• roltfl•u assent on 10 June 1996, and 0/tlh.g• sation, that Tokelau did not 1- •, Aotum•- Uu.t (Sw•ln•J · came into force on 1 August 1 f .. • Tllloplol ~~~~~ !•J.. ·-~~~oa wish to change its status ~ ~ 1996 - is but one piece in ' \, vis-a-vis New Zealand. the colourful mosaic of •l . However, in an unexpected Tokelau's constitutional de change of position (stimu- velopment. lated no doubt by external The colonialism that factors such as the UN pro Tokelau has known has posal to complete its been the British version, and decolonisation business by it has lasted so far for little the year 2000), the Ulu of over a century. -

British Virgin Islands
British Virgin Islands Clive Petrovic, Esther Georges and Nancy Woodfield Andy McGowan Great Tobago General introduction The British Virgin Islands comprise more than 60 islands, and the Virgin Islands. These include the globally cays and rocks, with a total land area of approximately 58 threatened Cordia rupicola (CR), Maytenus cymosa (EN) and square miles (150 square km). This archipelago is located Acacia anegadensis (CR). on the Puerto Rican Bank in the north-east Caribbean at A quarter of the 24 reptiles and amphibians identified are approximately 18˚N and 64˚W. The islands once formed a endemic, including the Anegada Rock Iguana Cyclura continuous land mass with the US Virgin Islands and pinguis (CR), which is now restricted to Anegada. Other Puerto Rico, and were isolated only in relatively recent endemics include Anolis ernestwilliamsii, Eleutherodactylus geologic time. With the exception of the limestone island of schwartzi, the Anegada Ground Snake Alsophis portoricensis Anegada, the islands are volcanic in origin and are mostly anegadae, the Virgin Gorda Gecko Sphaerodactylus steep-sided with rugged topographic features and little flat parthenopian, the Virgin Gorda Worm Snake Typlops richardi land, surrounded by coral reefs. naugus, and the Anegada Worm Snake Typlops richardi Situated at the eastern end of the Greater Antilles chain, the catapontus. Other globally threatened reptiles within the islands experience a dry sub-tropical climate dominated by BVI include the Anolis roosevelti (CR) and Epicrates monensis the prevailing north-east trade winds. Maximum summer granti (EN). temperatures reach 31˚C; minimum winter temperatures Habitat alteration during the plantation era and the are 19˚C, and there is an average rainfall of 700 mm per introduction of invasive alien species has had major year with seasonal hurricane events. -

UK Overseas Territories
INFORMATION PAPER United Kingdom Overseas Territories - Toponymic Information United Kingdom Overseas Territories (UKOTs), also known as British Overseas Territories (BOTs), have constitutional and historical links with the United Kingdom, but do not form part of the United Kingdom itself. The Queen is the Head of State of all the UKOTs, and she is represented by a Governor or Commissioner (apart from the UK Sovereign Base Areas that are administered by MOD). Each Territory has its own Constitution, its own Government and its own local laws. The 14 territories are: Anguilla; Bermuda; British Antarctic Territory (BAT); British Indian Ocean Territory (BIOT); British Virgin Islands; Cayman Islands; Falkland Islands; Gibraltar; Montserrat; Pitcairn, Henderson, Ducie and Oeno Islands; Saint Helena, Ascension and Tristan da Cunha; South Georgia and the South Sandwich Islands; Turks and Caicos Islands; UK Sovereign Base Areas. PCGN recommend the term ‘British Overseas Territory Capital’ for the administrative centres of UKOTs. Production of mapping over the UKOTs does not take place systematically in the UK. Maps produced by the relevant territory, preferably by official bodies such as the local government or tourism authority, should be used for current geographical names. National government websites could also be used as an additional reference. Additionally, FCDO and MOD briefing maps may be used as a source for names in UKOTs. See the FCDO White Paper for more information about the UKOTs. ANGUILLA The territory, situated in the Caribbean, consists of the main island of Anguilla plus some smaller, mostly uninhabited islands. It is separated from the island of Saint Martin (split between Saint-Martin (France) and Sint Maarten (Netherlands)), 17km to the south, by the Anguilla Channel. -

ATOLL RESEARCH Bulletln
ATOLL RESEARCH BULLETlN NO. 235 Issued by E SMTPISONIAIV INSTITUTION Washington, D.C., U.S.A. November 1979 CONTENTS Abstract Introduction Environment and Natural History Situation and Climate People Soils and Vegetation Invertebrate Animals Vertebrate Animals Material and Methods Systematics of the Land Crabs Coenobitidae Coenobi ta Coenobi ta brevimana Coenobi ta per1 a ta Coenobi ta rugosa Birgus Birgus latro Grapsidae Geogxapsus Geograpsus crinipes Geograpsus grayi Metopograpsus Metopograpsus thukuhar Sesarma Sesarma (Labuaniurn) ?gardineri ii Gecarcinidae page 23 Cardisoma 2 4 Cardisoma carnif ex 2 5 Cardisoma rotundum 2 7 Tokelau Names for Land Crabs 30 Notes on the Ecology of the Land Crabs 37 Summary 4 3 Acknowledgements 44 Literature Cited 4 5 iii LIST OF FIGURES (following page 53) 1. Map of Atafu Atoll, based on N.Z. Lands and Survey Department Aerial Plan No. 1036/7~(1974) . 2. Map of Nukunonu Atoll, based on N.Z. Lands and Survey Department Aerial Plan No. 1036/7~sheets 1 and 2 (1974). 3. Map of Fakaofo Atoll, based on N.Z. Lands and Survey Department Aerial Plan No. 1036/7C (1974). 4. Sesarma (Labuanium) ?gardineri. Dorsal view of male, carapace length 28 rnm from Nautua, Atafu. (Photo T.R. Ulyatt, National Museum of N. Z.) 5. Cardisoma carnifex. Dorsal view of female, carapace length 64 mm from Atafu. (Photo T.R. Ulyatt) 6. Cardisoma rotundurn. Dorsal view of male, carapace length 41.5 mm from Village Motu, Nukunonu. (Photo T.R. Ulyatt) LIST OF TABLES 0 I. Surface temperature in the Tokelau Islands ( C) Page 5 11. Mean rainfall in the Tokelau Islands (mm) 6 111, Comparative list of crab names from the Tokelau Islands, Samoa, Niue and the Cook islands, 3 5 IV. -

Report of 16Th SPREP Meeting
Report of the 16th SPREP Meeting 13–16 September 2005 Apia, Samoa Meeting Report SPREP IRC Cataloguing-in-Publication Data SPREP Meeting (16th : 2005 : Apia, Samoa) Report of the Sixteenth SPREP Meeting, 13 - 16 September 2005, Apia, Samoa. - Apia, Samoa : SPREP, 2005. 82 p. ; 29 cm. ISBN: 978-982-04-0309-3 1. Environmental policy - Oceania - Congresses. 2. Conservation of natural resources - Oceania - Congresses. 3. Environmental protection - Oceania - Congresses. I. Pacific Regional Environment Programme. II. Secretariat of the Pacific Regional Environment Programme. III. Title. 363.7099 Prepared for publication, and reproduced, in October 2005 by the Secretariat of the Pacific Regional Environment Programme (SPREP) PO Box 240, Apia, Samoa P: (685) 21929, F: (685) 20231, E: [email protected], W: www.sprep.org (C) Secretariat of the Pacific Regional Environment Programme (SPREP) Reproduction of this material, in whole or in part, in any form, is authorised provided appropriate acknowledgement of the source is given. Original text: English Report of the 16th SPREP Meeting 13–16 September 2005 Apia, Samoa Secretariat of the Pacific Regional Environment Programme PO Box 240, Vailima, Apia, Samoa T: (685) 21 929 F: (685) 20 231 E: [email protected] W: www.sprep.org Contents Report of the 16th SPREP Meeting 1 Agenda Item 1: Official Opening 1 Agenda Item 2: Appointment of Chair and Vice-Chair 4 Agenda Item 3: Adoption of Agenda and Working Procedures 4 Agenda Item 4: Action Taken on Matters Arising from Fifteenth SPREP Meeting 4 Agenda Item -

Hold Fast to the Treasures of Tokelau; Navigating Tokelauan Agency in the Homeland and Diaspora
1 Ke Mau Ki Pale O Tokelau: Hold Fast To The Treasures of Tokelau; Navigating Tokelauan Agency In The Homeland And Diaspora A PORTFOLIO SUBMITTED TO THE GRADUATE DIVISION OF THE UNIVERSITY OF HAWAI’I IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS IN PACIFIC ISLANDS STUDIES AUGUST 2014 BY Lesley Kehaunani Iaukea PORTFOLIO COMMITTEE: Terence Wesley-Smith, Chairperson David Hanlon John Rosa 2 © 2014 Lesley Kehaunani Iaukea 3 We certify that we have read this portfolio and that, in our opinion, it is satisfactory in scope and quality as a portfolio for the degree of Master of Arts in Pacific Islands Studies. _____________________________ Terence Wesley-Smith Chairperson ______________________________ David Hanlon ______________________________ John Rosa 4 Table of Contents Table of Contents 4 Acknowledgements 6 Chapter One: Introduction 8 1. Introduction 8 2. Positionality 11 3. Theoretical Framework 13 4. Significance 14 5. Chapter outline 15 Chapter Two: Understanding Tokelau and Her People 18 1. Tokelau and her Atolls 20 2. Story of Creation from abstract elements 21 3. Na Aho O Te Pohiha (The days of darkness) 21 4. Peopling of the Tokelau Atolls 23 5. Path of Origin 24 6. Fakaofo 25 7. Nukunonu 26 8. Atafu 26 9. Olohega 26 10. Olohega meets another fate 27 11. Western contact 30 12. Myth as Practice 31 Chapter Three: Cultural Sustainability Through an Educational Platform 33 1. Education in Tokelau 34 2. The Various Methods Used 37 3. Results and impacts achieved from this study 38 4. Learning from this experience 38 5. Moving forward 43 6. -

Final Project Completion Reports Are Made Available on Our Web Site, and Publicized in Our Newsletter and Other Communications
Organization Legal Name: Conservation International - Pacific Islands Program Project Title: Small Grants Mechanism for Polynesia-Micronesia Date of Report: 24 February 2014 Leilani Duffy Report Author and Contact CI Pacific Islands Information Apia - SAMOA CEPF Region: Polynesia-Micronesia Strategic Direction: 1-2-3 Grant Amount: $824,955 Project Dates: May 1, 2008-May 31, 2013 Implementation Partners for this Project (please explain the level of involvement for each partner): The following partners assisted CI-Pacific Islands in the implementation of the Small Grants Mechanism by participating as the Technical Advisory Committee in reviewing and providing advice on the project proposals received from potential grantees. These committee comprised of members from inter-governmental organizations, international NGOs, academic institutions, regional NGOs and UN Agencies. 1. Mr. Greg Sherley (served as the Chair of the TAG) UNEP Task Force Manager Pacific Region - Samoa 2. Easter Galuvao Biodiversity Advisor Secretariat Pacific Regional Programme, Samoa 3. Jean-Yves Meyer Environment Research Department French Polynesia 4. Mark O’Brien Pacific Birdlife International Fiji 5. Willie Kostka Micronesia Conservation Trust FSM 6. Marika Tuiwawa University South Pacific Fiji 7. Souad Boudjelas Pacific Invasive Initiative, Auckland NZ. Conservation Impacts Please explain/describe how your project has contributed to the implementation of the CEPF ecosystem profile. Please summarize the overall results/impact of your project. The CEPF Small Grants (SG) investment managed by CI-Pacific Islands for terrestrial biodiversity conservation in the Polynesia-Micronesia hotspot had addressed some issues and concern that were not directly covered in the large grants. The SG focused on the three strategic directions (SD) highlighted in the Ecosystem Profile which includes invasive species control, management and eradication; improved management of KBA sites and community awareness and species recovery. -

Index for Volumes 17-18
INDEX, VOLUMES 17-18 Includes four issues per volume. Index compiled by Russell T. Clement and Robert S. Means. AUTHOR INDEX Ahlburg, Dennis A. “Return Migration from the United States to American Samoa: Evidence from the 1980 and 1990 Censuses.” 17 (2): 71-84. Aldrich, Robert. France and the South Pacific since 1940. Reviewed by Paul de Deckker, Colin Newbury, and Michel Panoff. In “Book Review Forum,” 18 (3): 119-133. -------. Response to reviews of France and the South Pacific since 1940. In “Book Review Forum,” 18 (3): 133-142. Ariki, Pa Tuterangi. See Davis Augé, Marc. Review of Inalienable Possessions: The Paradox of Keeping- While-Giving, by Annette B. Weiner. In “Book Review Forum,” 18 (1): 114-118. Ballekom, Manu van, and Ray Harlow. Te Waiatatanga mai o te Atua: South Island Traditions Recorded by Matiaha Tiramorehu. Reviewed by John Charlot. In “Some Recent Publications of Maori Texts,” 18 (2): 139-149. Barker, Judith C. “Home Alone: The Effects of Out-Migration on Niuean Elders’ Living Arrangements and Social Supports.” 17 (3): 41-81. Beaudet, Jean-Michel, and Lionel Weiri, comps., text by Jean-Michel Beau- det and Kaloonbat Tein. Chants Kanaks: Cérémonies et berceuses. Reviewed by Peter Russell Crowe. In “Melanesian Music on Compact Disc: Some Significant Issues,” 18 (3): 147-159. Pacific Studies, Vol. 19, No. 1--March 1996 195 196 Pacific Studies, Vol. 19, No. 1--March 1996 Beechert, Edward D. See Lal, Munro, and Beechert Besnier, Niko. “The Truth and Other Irrelevant Aspects of Nukulaelae Gossip.” 17 (3): 1-39. Black, Peter W. “The Domestication of Catholicism on Tobi.” 17 (1): 1-28. -

REVISED DRAFT Round-Leaved Chaff Flower (Achyranthes Splendens Var
REVISED DRAFT Round-Leaved Chaff Flower (Achyranthes splendens var. rotundata) Habitat Conservation Plan Kenai Industrial Park Project Prepared for: Hawai‘i Department of Land and Natural Resources Applicant: CIRI Land Development Company 2525 C Street, Suite 500 Anchorage, Alaska 99503 Draft prepared by: AMEC Environment & Infrastructure, Inc. 23049 Ualena Street, Suite 1100 Honolulu, Hawai‘i 96819 Revisions and final document produced by: SWCA Environmental Consultants, Honolulu Office Bishop Square: ASB Tower 1001 Bishop Street, Suite 2800 Honolulu, Hawai‘i 96813 June 2013 REVISED DRAFT Round-Leaved Chaff Flower HCP; Kenai Industrial Park This page intentionally left blank 2013 SWCA Environmental Consultants REVISED DRAFT ROUND-LEAVED CHAFF FLOWER (ACHYRANTHES SPLENDENS VAR. ROTUNDATA) HABITAT CONSERVATION PLAN KENAI INDUSTRIAL PARK PROJECT PREPARED FOR: Hawaiʻi Department of Land and Natural Resources APPLICANT: CIRI Land Development Company 2525 C Street, Suite 500 Anchorage, Alaska 99503 DRAFT PREPARED BY: AMEC Environment & Infrastructure, Inc. 3049 Ualena Street, Suite 1100 Honolulu, HI. 96819 REVISIONS AND FINAL DOCUMENT PRODUCED BY: SWCA Environmental Consultants Honolulu Office Bishop Square: ASB Tower 1001 Bishop Street, Suite 2800 Honolulu, HI. 96813 June 2013 REVISED DRAFT Round-Leaved Chaff Flower HCP; Kenai Industrial Park This page intentionally left blank 2013 SWCA Environmental Consultants REVISED DRAFT Round-Leaved Chaff Flower HCP; Kenai Industrial Park TABLE OF CONTENTS 1 INTRODUCTION AND PROJECT OVERVIEW............................................................................