Population and Community Dynamics of Freshwater Decapods in Response to Ecological and Anthropogenic Factors in Subtropical Streams in the Caribbean
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Classification of Living and Fossil Genera of Decapod Crustaceans
RAFFLES BULLETIN OF ZOOLOGY 2009 Supplement No. 21: 1–109 Date of Publication: 15 Sep.2009 © National University of Singapore A CLASSIFICATION OF LIVING AND FOSSIL GENERA OF DECAPOD CRUSTACEANS Sammy De Grave1, N. Dean Pentcheff 2, Shane T. Ahyong3, Tin-Yam Chan4, Keith A. Crandall5, Peter C. Dworschak6, Darryl L. Felder7, Rodney M. Feldmann8, Charles H. J. M. Fransen9, Laura Y. D. Goulding1, Rafael Lemaitre10, Martyn E. Y. Low11, Joel W. Martin2, Peter K. L. Ng11, Carrie E. Schweitzer12, S. H. Tan11, Dale Tshudy13, Regina Wetzer2 1Oxford University Museum of Natural History, Parks Road, Oxford, OX1 3PW, United Kingdom [email protected] [email protected] 2Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007 United States of America [email protected] [email protected] [email protected] 3Marine Biodiversity and Biosecurity, NIWA, Private Bag 14901, Kilbirnie Wellington, New Zealand [email protected] 4Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, Taiwan, Republic of China [email protected] 5Department of Biology and Monte L. Bean Life Science Museum, Brigham Young University, Provo, UT 84602 United States of America [email protected] 6Dritte Zoologische Abteilung, Naturhistorisches Museum, Wien, Austria [email protected] 7Department of Biology, University of Louisiana, Lafayette, LA 70504 United States of America [email protected] 8Department of Geology, Kent State University, Kent, OH 44242 United States of America [email protected] 9Nationaal Natuurhistorisch Museum, P. O. Box 9517, 2300 RA Leiden, The Netherlands [email protected] 10Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th and Constitution Avenue, Washington, DC 20560 United States of America [email protected] 11Department of Biological Sciences, National University of Singapore, Science Drive 4, Singapore 117543 [email protected] [email protected] [email protected] 12Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. -

Amphidromy in Shrimps: a Life Cycle Between Rivers and the Sea
Lat. Am. J. Aquat. Res., 41(4): 633-650, 2013 Amphidromy in shrimps: a life cycle 633 “Studies on Freshwater Decapods in Latin America” Ingo S. Wehrtmann & Raymond T. Bauer (Guest Editors) DOI: 103856/vol41-issue4-fulltext-2 Review Amphidromy in shrimps: a life cycle between rivers and the sea Raymond T. Bauer1 1Department of Biology, University of Louisiana at Lafayette, Lafayette, Louisiana, 70504-2451 USA ABSTRACT. Amphidromy is a diadromous life history pattern, common in tropical and subtropical freshwater caridean shrimps, in which adults live, breed and spawn small-sized embryos in freshwater but have extended larval development (ELD) in marine waters. Most completely freshwater species spawn large embryos with either direct or abbreviated larval development (ALD). An important benefit of amphidromy is dispersal among river systems via marine larvae, which increases their access to alternative habitats. Thus, amphidromous species have much broader geographic distributions than closely related completely freshwater ones with ALD. ALD and freshwater ELD species appear to have evolved from amphidromous species with marine ancestors. Delivery of larvae to the sea in many amphidromous species is accomplished by upstream hatching and river drift of larvae to the sea. In other species, the females themselves apparently migrate down to marine waters to spawn. After development, the postlarvae must find a river mouth and migrate upstream to the adult habitat. Migrations occur at night, with juveniles swimming or crawling along the river or stream bank. Larvae are released during the wet or flood season of the year, while juvenile migrations take place during the dry or low-flow season. -

Rowley, Marissa (2013) Interspecific Competition Between Prawns in The
Interspecific Competition between Prawns in the Checkhall River Marissa Rowley Dominica 2013 May 18 – June 9 ABSTRACT On the island of Dominica, nature remains virtually untouched by humans. This makes the island an exceptional choice for biological research as well as animal habitats. With its many different ecosystems, numerous animals take refuge throughout the island. Five species of prawns coexist within the Checkhall River on the island of Dominica, two of which lack pincers. In the Checkhall River, I researched competition of prawns for available food sources in different areas of the stream, including shallow areas, deep areas, and waterfall areas. Coconut was tied to fishing line, weighted down with a rock, and used as bait. The bait line was then dropped in chosen areas of the stream and watched for interspecific interactions until a clear winner was present. Multiple trials were held in each designated spot. From the results, it is clear the Macrobrachium species of prawns dominate competition in the Checkhall River. I believe this is because of their larger pincers. Their pincers can be used to obtain and keep hold of a food source and intimidate other species of prawns. This research can be helpful to future biologists studying the behavioral ecology of freshwater prawns. INTRODUCTION Dominica is a relatively small volcanic island located in the Lesser Antillean chain of the Caribbean. Appropriately nicknamed the Nature Island, Dominica is characterized by many different habitats including mountainous terrain, rainforests, waterfalls, lakes, and rivers (Discover Dominica Authority). These different ecosystems make Dominica an exceptional place for field biology research as they remain mostly untouched by humans and modern society. -
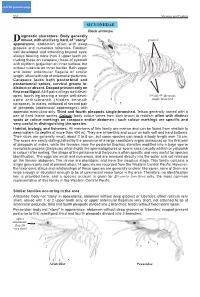
W7192e19.Pdf
click for previous page 952 Shrimps and Prawns Sicyoniidae SICYONIIDAE Rock shrimps iagnostic characters: Body generally Drobust, with shell very hard, of “stony” grooves appearance; abdomen often with deep grooves and numerous tubercles. Rostrum well developed and extending beyond eyes, always bearing more than 3 upper teeth (in- cluding those on carapace); base of eyestalk with styliform projection on inner surface, but without tubercle on inner border. Both upper and lower antennular flagella of similar length, attached to tip of antennular peduncle. 1 Carapace lacks both postorbital and postantennal spines, cervical groove in- distinct or absent. Exopod present only on first maxilliped. All 5 pairs of legs well devel- 2 oped, fourth leg bearing a single well-devel- 3rd and 4th pleopods 4 single-branched oped arthrobranch (hidden beneath 3 carapace). In males, endopod of second pair 5 of pleopods (abdominal appendages) with appendix masculina only. Third and fourth pleopods single-branched. Telson generally armed with a pair of fixed lateral spines. Colour: body colour varies from dark brown to reddish; often with distinct spots or colour markings on carapace and/or abdomen - such colour markings are specific and very useful in distinguishing the species. Habitat, biology, and fisheries: All members of this family are marine and can be found from shallow to deep waters (to depths of more than 400 m). They are all benthic and occur on both soft and hard bottoms. Their sizes are generally small, about 2 to 8 cm, but some species can reach a body length over 15 cm. The sexes are easily distinguished by the presence of a large copulatory organ (petasma) on the first pair of pleopods of males, while the females have the posterior thoracic sternites modified into a large sperm receptacle process (thelycum) which holds the spermatophores or sperm sacs (usually whitish or yellowish in colour) after mating. -

Melanoides Tuberculata), Species Habitat Associations and Life History Investigations in the San Solomon Spring Complex, Texas
FINAL REPORT As Required by THE ENDANGERED SPECIES PROGRAM TEXAS Grant No. TX E-121-R Endangered and Threatened Species Conservation Native springsnails and the invasive red-rim melania snail (Melanoides tuberculata), species habitat associations and life history investigations in the San Solomon Spring complex, Texas Prepared by: David Rogowski Carter Smith Executive Director Clayton Wolf Director, Wildlife 3 October 2012 FINAL REPORT STATE: ____Texas_______________ GRANT NUMBER: ___ TX E-121-R___ GRANT TITLE: Native springsnails and the invasive red-rim melania snail (Melanoides tuberculata), species habitat associations and life history investigations in the San Solomon Spring complex, Texas. REPORTING PERIOD: ____17 Sep 09 to 31 May 12_ OBJECTIVE(S): To determine patterns of abundance, distribution, and habitat use of the Phantom Cave snail (Cochliopa texana), Phantom Spring tryonia (Tryonia cheatumi), and the invasive red-rim melania snail (Melanoides tuberculta) in San Solomon Springs, and potential interactions. Segment Objectives: Task 1. January - February 2010. A reconnaissance visit(s) will be made to the region to investigate the study area and work on specific sampling procedural methods. Visit with TPWD at the Balmorhea State Park, as well as meet The Nature Conservancy personnel at Diamond Y and Sandia springs complexes. Task 2. March 2010– August 2011. Begin sampling. Field sampling will be conducted every 6-8 weeks, over a period of a year and a half. Sampling methods are outlined below stated Tasks. Task 3. December 2010. Completion of first year of study. With four seasonal samples completed, preliminary data analysis and statistical modeling will begin. Preliminary results will be presented at the Texas Chapter of the American Fisheries Society meeting. -

Evolutionary Genomics of a Plastic Life History Trait: Galaxias Maculatus Amphidromous and Resident Populations
EVOLUTIONARY GENOMICS OF A PLASTIC LIFE HISTORY TRAIT: GALAXIAS MACULATUS AMPHIDROMOUS AND RESIDENT POPULATIONS by María Lisette Delgado Aquije Submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy at Dalhousie University Halifax, Nova Scotia August 2021 Dalhousie University is located in Mi'kma'ki, the ancestral and unceded territory of the Mi'kmaq. We are all Treaty people. © Copyright by María Lisette Delgado Aquije, 2021 I dedicate this work to my parents, María and José, my brothers JR and Eduardo for their unconditional love and support and for always encouraging me to pursue my dreams, and to my grandparents Victoria, Estela, Jesús, and Pepe whose example of perseverance and hard work allowed me to reach this point. ii TABLE OF CONTENTS LIST OF TABLES ............................................................................................................ vii LIST OF FIGURES ........................................................................................................... ix ABSTRACT ...................................................................................................................... xii LIST OF ABBREVIATION USED ................................................................................ xiii ACKNOWLEDGMENTS ................................................................................................ xv CHAPTER 1. INTRODUCTION ....................................................................................... 1 1.1 Galaxias maculatus .................................................................................................. -

Effects of Drought and Hurricane Disturbances on Headwater Distributions of Palaemonid River Shrimp (Macrobrachium Spp.) in the Luquillo Mountains, Puerto Rico
J. N. Am. Benthol. Soc., 2006, 25(1):99–107 Ó 2006 by The North American Benthological Society Effects of drought and hurricane disturbances on headwater distributions of palaemonid river shrimp (Macrobrachium spp.) in the Luquillo Mountains, Puerto Rico Alan P. Covich1 Institute of Ecology, University of Georgia, Athens, Georgia 30602-2202 USA 2 3 Todd A. Crowl AND Tamara Heartsill-Scalley Ecology Center and Department of Aquatic, Watershed, and Earth Sciences, Utah State University, Logan, Utah 84322 USA Abstract. Extreme events (hurricanes, floods, and droughts) can influence upstream migration of macroinvertebrates and wash out benthic communities, thereby locally altering food webs and species interactions. We sampled palaemonid river shrimp (Macrobrachium spp.), dominant consumers in headwaters of the Luquillo Mountains of northeastern Puerto Rico, to determine their distributions along an elevational gradient (274–456 m asl) during a series of disturbances (Hurricane Hugo in 1989, a drought in 1994, and Hurricane Georges in 1998) that occurred over a 15-y period (1988À2002). We measured shrimp abundance 3 to 6 times/y in Quebrada Prieta in the Espiritu Santo drainage as part of the Luquillo Long- Term Ecological Research Program. In general, Macrobrachium abundance declined with elevation during most years. The lowest mean abundance of Macrobrachium occurred during the 1994 drought, the driest year in 28 y of record in the Espiritu Santo drainage. Macrobrachium increased in abundance for 6 y following the 1994 drought. In contrast, hurricanes and storm flows had relatively little effect on Macrobrachium abundance. Key words: dispersal, drainage networks, geomorphology, habitat preference, omnivores, prey refugia. Flow-based events often modify the important roles (Covich et al. -

A Migratory Shrimp's Perspective on Habitat Fragmentation in The
A MIGRATORY SHRIMP’S PERSPECTIVE ON HABITAT FRAGMENTATION IN THE NEOTROPICS: EXTENDING OUR KNOWLEDGE FROM PUERTO RICO BY MARCIA N. SNYDER1,3), ELIZABETH P. ANDERSON2,4) and CATHERINE M. PRINGLE1,5) 1) Odum School of Ecology, University of Georgia, Athens, Georgia 30602, U.S.A. 2) Global Water for Sustainability Program, Florida International University, Miami, FL 33199, U.S.A. ABSTRACT Migratory freshwater fauna depend on longitudinal connectivity of rivers throughout their life cycles. Amphidromous shrimps spend their adult life in freshwater but their larvae develop into juveniles in salt water. River fragmentation resulting from pollution, land use change, damming and water withdrawals can impede dispersal and colonization of larval shrimps. Here we review current knowledge of river fragmentation effects on freshwater amphidromous shrimp in the Neotropics, with a focus on Puerto Rico and Costa Rica. In Puerto Rico, many studies have contributed to our knowledge of the natural history and ecological role of migratory neotropical shrimps, whereas in Costa Rica, studies of freshwater migratory shrimp have just begun. Here we examine research findings from Puerto Rico and the applicability of those findings to continental Costa Rica. Puerto Rico has a relatively large number of existing dams and water withdrawals, which have heavily fragmented rivers. The effects of fragmentation on migratory shrimps’ distribution have been documented on the landscape-scale in Puerto Rico. Over the last decade, dams for hydropower production have been constructed on rivers throughout Costa Rica. In both countries, large dams restrict shrimps from riverine habitat in central highland regions; in Puerto Rico 27% of stream kilometers are upstream of large dams while in Costa Rica 10% of stream kilometers are upstream of dams. -

Los Camarones Del Bosque Nacional El Yunque
i te fijas bien en los ríos y las pozas Pueden nadar rápidamente hacia atrás por cortos Y...¿Cómo se reproducen los camarones ? de El Yunque puedes que veas algo periódos de tiempo haciendo un rápido moverse rápidamente por el fondo. movimiento o flexión en el abdomen. con el rabo. La reproducción y etapas de la vida de un Lo más probable hayas visto una de camarón son muy inetersantes. La época Slas 10 especies de camarones que habitan en Las etapas de la vida de un camarón son muy reproductiva de los camarones ocurre durante las aguas del interesantes. El camarón adulto vive en charcas y los meses de verano aunque hay especies que se bosque. La mayoría rápidos y por la noche se concentran sobre reproducen durante todo el año. de los sistemas de piedras, ramas de árboles y hojas pues es un agua dulce y animal de hábitos nocturnos. El apareamiento se lleva a cabo cuando el salobre de Puerto macho se coloca en ángulo recto con la hembra Rico están Micratya poeyi Especies y Hábitos alimenticios y le transfiere un espermatóforo a un habitados por receptáculo en el abdomen de la hembra. De 6 a camarones de las familias Atyidae y En Puerto Rico existen 9 especies de la familia 20 horas después del apareamiento, la hembra Palaemonidae. Atyidae y de éstas seis 6 se encuentran en los ríos carga bajo su abdomen una gran cantidad de de El Yunque. huevos. Esta cantidad depende de la especie y Estas curiosas especies de animales tienen Los miembros de el individuo. -

Epilobocera Wetherbeei, a New Species of Freshwater Crab (Decapoda: Brachyura: Pseudothelphusidae) from Hispaniola Gilberto Rodr
PROCEEDINGS OF THE BIOLOGICAL SOCIETY OF WASHINGTON 108(l):76-83. 1995. Epilobocera wetherbeei, a new species of freshwater crab (Decapoda: Brachyura: Pseudothelphusidae) from Hispaniola Gilberto Rodriguez and Austin B. Williams (GR) Instituto Venezolano de Investigaciones Cientificas, Apartado 21827, Caracas 1020 A, Venezuela; (ABW) National Marine Fisheries Service Systematics Laboratory, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560, U.S.A. Abstract.—Epilobocera wetherbeei, a new species of pseudothelphusid crab, is described from the Dominican Republic. The species can be easily distin guished from other species of Epilobocera by its small size, absence of antero lateral spinulation and characters of the first male gonopods. SEM micropho- tographs of the first and second male gonopods of E. wetherbeei, E. sinuatifrons, E. haytensis and E. gertraudae are provided. The apex of the second male gonopod in all species of Epilobocera studied is hollow, transversely truncate, with a prominent internal rib provided with spines. These characters distinctly separate Epilobocera from species in the subfamily Pseudothelphusinae where the gonopodal apex is spoon-shaped and provided with long setae. The new observations are incorporated into amended diagnoses of the subfamilies Epi- lobocerinae and Pseudothelphusinae. The genus Epilobocera Stimpson, 1860, less it can be shown to be a synonym of E. comprises six species of freshwater crabs re armata."' The genus has been the object of stricted to the Greater Antilles and some recent revisions by Chace & Hobbs (1969), nearby islands: E. sinuatifrons A. Milne Ed Pretzmann (1972) and Rodriguez (1982). wards, 1866, inhabits Puerto Rico and Saint Due to the accessibility and small area of Croix Island; E. -

Alien Tropical Snail, the Red-Rimmed Melania Melanoides Tuberculata Müller, 1774 (Thiaridae) from Artificial Lake in Warsaw, Central Poland
Available online at www.worldscientificnews.com WSN 132 (2019) 285-290 EISSN 2392-2192 SHORT COMMUNICATION Alien tropical snail, the red-rimmed melania Melanoides tuberculata Müller, 1774 (Thiaridae) from artificial lake in Warsaw, Central Poland Rafał Maciaszek1,*, Dorota Marcinek2, Witold Sosnowski3 1 Department of Genetics and Animal Breeding, Faculty of Animal Sciences, Warsaw University of Life Sciences, ul. Ciszewskiego 8, 02-786 Warsaw, Poland 2 Faculty of Animal Sciences, Warsaw University of Life Sciences, ul. Ciszewskiego 8, 02-786 Warsaw, Poland 3Laboratory of Marine Organisms Reproduction, Faculty of Food Sciences and Fisheries, West Pomeranian University of Technology, ul. Kazimierza Królewicza 4, 70-001, Szczecin, Poland *E-mail address: [email protected] ABSTRACT This paper describes the case of introduction of an exotic ornamental snail, Melanoides tuberculata (Thiaridae) in an artificial water reservoir in Pole Mokotowskie park complex in Warsaw, Poland. Observed individuals have been identified, described and presented in photographs. The basic physicochemical parameters of water were analyzed and prospects for the population were evaluated. Another second species of aquatic ornamental snail - Physa acuta (Physidae) was found in the same water reservoir. The observation was analyzed with available literature describing introductions of alien species of aquatic origin in Polish waters. Keywords: aquarium, invasive species, freshwater mollusc, ornamental pet, tadpole snail, Pole Mokotowskie park complex, Melanoides tuberculata ( Received 12 July 2019; Accepted 27 July 2019; Date of Publication 28 July 2019 ) World Scientific News 132 (2019) 285-290 1. INTRODUCTION The red-rimmed melania, Melanoides tuberculata (Müller, 1774) is a viable, parthenogenetic species of snail that naturally inhabits freshwaters of southern Asia, Africa, Madagascar and northern Australia. -

A Dissertation Entitled Evolution, Systematics
A Dissertation Entitled Evolution, systematics, and phylogeography of Ponto-Caspian gobies (Benthophilinae: Gobiidae: Teleostei) By Matthew E. Neilson Submitted as partial fulfillment of the requirements for The Doctor of Philosophy Degree in Biology (Ecology) ____________________________________ Adviser: Dr. Carol A. Stepien ____________________________________ Committee Member: Dr. Christine M. Mayer ____________________________________ Committee Member: Dr. Elliot J. Tramer ____________________________________ Committee Member: Dr. David J. Jude ____________________________________ Committee Member: Dr. Juan L. Bouzat ____________________________________ College of Graduate Studies The University of Toledo December 2009 Copyright © 2009 This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. _______________________________________________________________________ An Abstract of Evolution, systematics, and phylogeography of Ponto-Caspian gobies (Benthophilinae: Gobiidae: Teleostei) Matthew E. Neilson Submitted as partial fulfillment of the requirements for The Doctor of Philosophy Degree in Biology (Ecology) The University of Toledo December 2009 The study of biodiversity, at multiple hierarchical levels, provides insight into the evolutionary history of taxa and provides a framework for understanding patterns in ecology. This is especially poignant in invasion biology, where the prevalence of invasiveness in certain taxonomic groups could