The Small Intestine H.J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transcytosis of Listeria Monocytogenes Across The
Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin Georgios Nikitas, Chantal Deschamps, Olivier Disson, Théodora Niault, Pascale Cossart, Marc Lecuit To cite this version: Georgios Nikitas, Chantal Deschamps, Olivier Disson, Théodora Niault, Pascale Cossart, et al.. Tran- scytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin. Journal of Experimental Medicine, Rockefeller University Press, 2011, 208 (11), pp.2263-2277. 10.1084/jem.20110560. pasteur-02040395 HAL Id: pasteur-02040395 https://hal-pasteur.archives-ouvertes.fr/pasteur-02040395 Submitted on 20 Feb 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial - NoDerivatives| 4.0 International License Published Online: 3 October, 2011 | Supp Info: http://doi.org/10.1084/jem.20110560 Downloaded from jem.rupress.org on February 19, 2019 Article Transcytosis of Listeria monocytogenes across -

Gastrointestinal Motility
Gastrointestinal Motility H. J. Ehrlein and M.Schemann 1. Motility of the stomach Anatomic regions of the stomach are the fundus, corpus (body), antrum and pylorus. The functional regions of the stomach do not correspond to the anatomic regions. Functionally, the stomach can be divided into the gastric reservoir and the gastric pump (Fig. 1). The gastric reservoir consists of the fundus and corpus. The gastric pump is represented by the area at which peristaltic waves occur: it includes the distal part of the corpus and the antrum. Due to different properties of the smooth muscle cells the gastric reservoir is characterised by tonic activity and the gastric pump by phasic activity. AB Gastric reservoir Fundus tonic contractions Pylorus Corpus Antrum Gastric pump phasic contractions Figure 1 . The stomach can be divided into three anatomic (A) and two functional regions (B) 1.1 Function of the gastric reservoir At the beginning of the 20 th century it was already observed that with increasing volume of the stomach the internal pressure of the stomach increases only slightly. In dogs, for instance, the increase in pressure is only 1.2 cm of water/100 ml volume. The small increase in gastric pressure indicates that the stomach does not behave like an elastic balloon but that it relaxes as it fills. Three kinds of gastric relaxation can be differentiated: a receptive, an adaptive and a feedback-relaxation of the gastric reservoir. The receptive relaxation consists of a brief relaxation during chewing and swallowing. The stimulation of mechano-receptors in the mouth and pharynx induces vago-vagal reflexes which cause a relaxation of the gastric reservoir (Fig. -

Regulation of Intestinal Blood Flow
Journal of Surgical Research 93, 182–196 (2000) doi:10.1006/jsre.2000.5862, available online at http://www.idealibrary.com on RESEARCH REVIEW Regulation of Intestinal Blood Flow Paul J. Matheson, Ph.D.,*,†,1 Mark A. Wilson, M.D., Ph.D.,*,†,‡ and R. Neal Garrison, M.D.*,†,‡ *Center for Excellence in Applied Microcirculatory Research and ‡Department of Surgery, University of Louisville, Louisville, Kentucky 40292; and †Louisville Veterans Affairs Medical Center, Louisville, Kentucky 40206 Submitted for publication July 29, 1999 arteries is typically 20–25% of cardiac output in the The gastrointestinal system anatomically is posi- unfed state [2]. There is extensive overlap or collateral tioned to perform two distinct functions: to digest and circulation in the distal vascular distributions of these absorb ingested nutrients and to sustain barrier func- arteries. During nutrient absorption, blood flow in each tion to prevent transepithelial migration of bacteria of these arteries is increased sequentially as the diges- and antigens. Alterations in these basic functions con- tive chyme passes over the mucosal surface supplied by tribute to a variety of clinical scenarios. These pri- mary functions intrinsically require splanchnic blood the particular arteries [3]. Following nutrient absorp- flow at both the macrovascular and microvascular lev- tion, the blood flow to each segment returns to baseline els of perfusion. Therefore, a greater understanding of levels as the chyme moves past that region of the the mechanisms that regulate intestinal vascular per- digestive tract [4, 5]. This postprandial increase in fusion in the normal state and during pathophysiolog- blood flow is independent of organ distention and is ical conditions would be beneficial. -
Topic 6.1 Answers
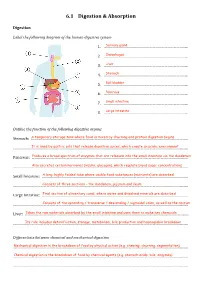
6.1 Digestion & Absorption Digestion Label the following diagram of the human digestive system 1. ………………………………………………………………………...Salivary gland 2. ………………………………………………………………………...Oesophagus 3. ………………………………………………………………………...Liver 4. ………………………………………………………………………...Stomach 5. ………………………………………………………………………...Gall bladder 6. ……Pancreas…………………………………………………………………... 7. ………………………………………………………………………...Small intestine 8. ………………………………………………………………………...Large intestine Outline the function of the following digestive organs Stomach: ………………………………………………………………………………………A temporary storage tank where food is mixed by churning and………………………………………………… protein digestion begins …………………………………………………………………………………………………………………………………………………..........It is lined by gastric pits that release digestive juices, which create an acidic environment Pancreas: …………..……………………………………………………………………………………………………………………………Produces a broad spectrum of enzymes that are released into the small intestine via the duodenum …………………………………………………………………………………………………………………………………………………..........Also secretes certain hormones (insulin, glucagon), which regulate blood sugar concentrations Small Intestine: …………….……………………………………………………………………………………………A long, highly folded tube where usable food substances (nutrients) are absorbed…………………… …………………………………………………………………………………………………………………………………………………..........Consists of three sections – the duodenum, jejunum and ileum Large Intestine: …………….…………………………………………………………………………………………………………………Final section of alimentary canal, where water and dissolved minerals are absorbed …………………………………………………………………………………………………………………………………………………..........Consists -

New Developments in Goblet Cell Mucus Secretion and Function
REVIEW nature publishing group New developments in goblet cell mucus secretion and function GMH Birchenough1, MEV Johansson1, JK Gustafsson1, JH Bergstro¨m1 and GC Hansson1 Goblet cells and their main secretory product, mucus, have long been poorly appreciated; however, recent discoveries have changed this and placed these cells at the center stage of our understanding of mucosal biology and the immunology of the intestinal tract. The mucus system differs substantially between the small and large intestine, although it is built around MUC2 mucin polymers in both cases. Furthermore, that goblet cells and the regulation of their secretion also differ between these two parts of the intestine is of fundamental importance for a better understanding of mucosal immunology. There are several types of goblet cell that can be delineated based on their location and function. The surface colonic goblet cells secrete continuously to maintain the inner mucus layer, whereas goblet cells of the colonic and small intestinal crypts secrete upon stimulation, for example, after endocytosis or in response to acetyl choline. However, despite much progress in recent years, our understanding of goblet cell function and regulation is still in its infancy. THE INTESTINE system of mucus covering the epithelium. There is a The gastrointestinal tract is a remarkable organ. Not only can it two-layered mucus system in the stomach and colon and a digest most of our food into small components, but it is also single-layered mucus in the small intestine.5 The mucus layers filled with kilograms of microbes that live in stable equilibrium in these three regions perform their protective function using with us and our immune system. -

The Transcriptional Repressor Blimp1/Prdm1 Regulates Postnatal Reprogramming of Intestinal Enterocytes
The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes James Harpera, Arne Moulda, Robert M. Andrewsb, Elizabeth K. Bikoffa, and Elizabeth J. Robertsona,1 aSir William Dunn School of Pathology, University of Oxford, Oxford OX1 3RE, United Kingdom; and bWellcome Trust Sanger Institute, Genome Campus, Hinxton-Cambridge CB10 1SA, United Kingdom Edited by Brigid L. M. Hogan, Duke University Medical Center, Durham, NC, and approved May 19, 2011 (received for review April 13, 2011) Female mammals produce milk to feed their newborn offspring rise to the developing crypts. The crypt derived adult enterocytes before teeth develop and permit the consumption of solid food. that emerge at postnatal day (P) 12 and repopulate the entire Intestinal enterocytes dramatically alter their biochemical signature epithelium lack Blimp1 expression. Conditional inactivation of during the suckling-to-weaning transition. The transcriptional re- Blimp1 results in globally misregulated gene expression patterns, pressor Blimp1 is strongly expressed in immature enterocytes in and compromises small intestine (SI) tissue architecture, nutrient utero, but these are gradually replaced by Blimp1− crypt-derived uptake, and survival of the neonate. In combination with tran- adult enterocytes. Here we used a conditional inactivation strategy scriptional profiling, the present experiments demonstrate that to eliminate Blimp1 function in the developing intestinal epithe- Blimp1/Prdm1 orchestrates orderly and extensive reprogramming lium. There was no noticeable effect on gross morphology or of the postnatal intestinal epithelium. formation of mature cell types before birth. However, survival of mutant neonates was severely compromised. Transcriptional Results profiling experiments reveal global changes in gene expression Down-Regulated Blimp1 Expression in Crypt Progenitors Beginning at patterns. -

Octreotide in Gastrointestinal Motility Disorders Gut: First Published As 10.1136/Gut.35.3 Suppl.S11 on 1 January 1994
Gut 1994; supplement 3: S11 -S 14 Sll Octreotide in gastrointestinal motility disorders Gut: first published as 10.1136/gut.35.3_Suppl.S11 on 1 January 1994. Downloaded from C Owyang Abstract Intestinal effects of octreotide in The effects of octreotide on six normal scleroderma subjects and five patients with sclero- About 50% of patients with scleroderma have derma were investigated. Changes in small bowel dysfunction.5 In such patients, intestinal motility and in plasma motilin manometry shows patterns (known as the were examined after a single injection of migrating motor complex) in the small bowel octreotide. Octreotide stimulated intense during fasting6 and this may be clinically intestinal motor activity in normal sub- manifested as intestinal pseudo-obstruction jects. Motility patterns in the scleroderma and bacterial overgrowth. These problems are patients were chaotic and non-pro- difficult to treat because standard stimulatory pagative, but, after octreotide was given, prokinetic agents are not effective in sclero- became well coordinated, aborally derma.6 We therefore recently undertook a directed, and nearly as intense as in study to determine the effects of octreotide in normal volunteers. Clinical responses and six normal subjects and in five patients with changes in breath hydrogen were also scleroderma who had abdominal pain, nausea, evaluated in the five scleroderma patients bloating, and a change in intestinal contrac- who had further treatment with octreotide tility.7 We examined the changes in intestinal at a dose of 50 pugtday subcutaneously for motility and in plasma motilin, a gastrointesti- three weeks. A reduction in symptoms of nal hormone that stimulates intestinal motor abdominal pain, nausea, vomiting, and activity, after single injections of octreotide bloating was seen. -

5-Hydroxytryptamine Andhuman Small Intestinal Motility
496 Gut 1994; 35:496-500 5-Hydroxytryptamine and human small intestinal motility: effect ofinhibiting 5-hydroxytryptamine reuptake D A Gorard, G W Libby, M J G Farthing Abstract nature is poorly understood.'0 In animals, Parenteral 5-hydroxytryptamine stimulates migrating motor complex cycling can be small intestinal motility, but the effect of con- modified by administration of 5-HT, its pre- tinuous stimulation with 5-hydroxytryptamine cursor 5-hydroxytryptophan, and its antago- on the human migrating motor complex is nists." '4 The effect of 5-HT on the migrating unknown. Using a selective 5-hydroxytrypta- motor complex in humans has not been studied. mine reuptake inhibitor, paroxetine, this study Tachyphylaxis to 5-HT given intravenously,45 investigated the effect of indirect 5-hydroxy- and associated cardiovascular and pulmonary tryptamine agonism on fasting small responses limit prolonged infusion of 5-HT in intestinal motility and transit. Eight healthy humans. The effect, however, of 5-HT agonism subjects were studied while receiving paroxe- on human small intestinal motor function might tine 30 mg daily for five days and while receiv- be alternatively investigated using a selective ing no treatment, in random order. Ambulant 5-HT reuptake inhibitor. Paroxetine selectively small intestinal motility was recorded from five inhibits the neuronal reuptake of 5-HT, increas- sensors positioned from the duodenojejunal ing the availability of synaptic 5-HT. Its ability flexure to the ileum for 16-18 hours. Paroxe- to inhibit 5-HT reuptake exceeds its ability to tine reduced the migrating motor complex inhibit noradrenaline reuptake by a factor of periodicity mean (SEM) from 81 (6) min to 67 320," making it four to five hundred times (4) min (p<005), and increased the propaga- as selective as the standard tricycic anti- tion velocity of phase III from 3-1 to 4-7 cm/ depressants imipramine and amitriptyline. -

Aandp2ch25lecture.Pdf
Chapter 25 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © McGraw-Hill Education. Permission required for reproduction or display. 1 Introduction • Most nutrients we eat cannot be used in existing form – Must be broken down into smaller components before body can make use of them • Digestive system—acts as a disassembly line – To break down nutrients into forms that can be used by the body – To absorb them so they can be distributed to the tissues • Gastroenterology—the study of the digestive tract and the diagnosis and treatment of its disorders 25-2 General Anatomy and Digestive Processes • Expected Learning Outcomes – List the functions and major physiological processes of the digestive system. – Distinguish between mechanical and chemical digestion. – Describe the basic chemical process underlying all chemical digestion, and name the major substrates and products of this process. 25-3 General Anatomy and Digestive Processes (Continued) – List the regions of the digestive tract and the accessory organs of the digestive system. – Identify the layers of the digestive tract and describe its relationship to the peritoneum. – Describe the general neural and chemical controls over digestive function. 25-4 Digestive Function • Digestive system—organ system that processes food, extracts nutrients, and eliminates residue • Five stages of digestion – Ingestion: selective intake of food – Digestion: mechanical and chemical breakdown of food into a form usable by -

The Number of Villi in Rat's Jejunum and Ileum: Effect of Normal Growth, Partial Enterectomy, and Tube Feeding
J. Anat. (1972). 111, 2, pp. 283-291 283 With 6 figures Printed in Great Britain The number of villi in rat's jejunum and ileum: effect of normal growth, partial enterectomy, and tube feeding J. M. FORRESTER Department of Physiology, Edinburgh University (Accepted 8 January 1972) INTRODUCTION The villi of the rat small intestine are covered by enterocytes which have a life-span, from their time of origin in the crypts until shedding at the villus tip, of only about one and a half days (Leblond & Stevens, 1948; Bertalanffy, 1960). Their shape varies from one part of the small intestine to another, and even adjacent villi may differ strikingly. In view of these features suggesting a rapidly changing scene, this paper describes a procedure for enumerating the villi in rat jejunum and ileum, and ex- amines the stability of the total number during normal growth, after partial enter- ectomy, and after tube feeding. METHODS Locally bred Wistar male rats were used. They were fed on a standard pelleted rat food manufactured in Edinburgh. They always had tap water ad libitum. Enumeration procedure. Rats were killed by inhalation of chloroform in the morn- ing. The position of the suspensory ligament was marked on the small intestine where a band of connective tissue is attached to the intestine at the duodenojejunal junction. Then the small intestine was removed from pylorus to ileocolic valve by gentle traction, and washed through with cold saline (NaCl 0-9 %, w/v). It was weighed, and after removal of the duodenum, was laid in a trough and perfused with Bouin's solution at an outlet pressure of 20 cm of solution for at least 20 minutes (Hromadkova & Skala, 1969). -

Enterocyte Proliferation and Intracellular Bacteria in Animals
Gut 1994; 35: 1483-1486 1483 PROGRESS REPORT Gut: first published as 10.1136/gut.35.10.1483 on 1 October 1994. Downloaded from Enterocyte proliferation and intracellular bacteria in animals S McOrist, C J Gebhart, G H K Lawson Considerable proliferation of enterocytes, candidates for the bacteria involved.2 Recent forming adenomatous growths within the work shows that the intracellular bacteria are a intestinal mucosa, is a consistent feature of a new genus of obligate intracellular bacteria, number of enteric conditions in animals, now living within enterocytes. This report describes referred to under the general heading of pro- recent advances in the taxonomic status of the liferative enteropathy. These were originally bacteria and its association with disease patho- described in pigs as adenomas ofthe ileum and genesis in animals, focusing on how this can colon1 and this condition was found to have a aid an understanding of enterocyte prolifera- worldwide occurrence.2 Similar conditions tion. were subsequently described in a number of other animal species, again under the general heading of proliferative enteropathy or Intracellular bacteria enteritis. The degree, however, of mucosal The intracellular bacteria in proliferative proliferation, corresponding to a description of enteropathy in animals have been closely hyperplasia, through adenoma to a more studied in the pig and hamster species, in carcinomatous form, varies between species. which the disease is common and widespread. Occasional reports of the condition in the fox,3 Clinical diagnosis of infection and disease is, horse,4 and guinea pig5 describe a hyperplastic however, difficult2 and the disease might be condition of the mucosa whereas hamsters6 7 recognised more widely if diagnostic tools develop adenomatous lesions similar to the became available. -

Effect of Chronic Ethanol Ingestion on Enterocyte Turnover in Rat Small Intestine
Gut: first published as 10.1136/gut.28.1.52 on 1 January 1987. Downloaded from Gut, 1987, 28, 52-55 Effect of chronic ethanol ingestion on enterocyte turnover in rat small intestine R MAZZANTI AND W J JENKINS From the Department of' Medicine, Royal Free Hospital School of Medicine, Rowland Hill Street, Londlon SUMMARY Whether chronic ethanol ingestion significantly damages the small intestine remains controversial. To clarify this we have analysed the morphology of the small intestinal epithelium and quantified its renewal in chronically ethanol fed rats. Twenty adult male rats were pair fed for 28 days a nutritionally adequate liquid diet containing either ethanol as 36% of total calories or an isocaloric diet in which fat substituted for ethanol. Crypt cell production rate was determined in the jejunum and ileum by the metaphase arrest method. Weight gain and small intestinal morphology were similar in ethanol fed and control rats, but enterocyte turnover was significantly reduced in the jejunum (p<O.05) and ileum (p<0.0l) of the ethanol fed rats. This effect of ethanol on the small intestine is probably systemic rather than local, because the changes in jejunum and ileum were similar, and it may contribute to the development of malnutrition in chronic alcoholics. A variety of changes in intestinal function have Methods been reported after acute or habitual alcohol consumption.' Impaired absorption of vitamins,25 ANIMALS sugars6 7 and amino acids8 9 has been shown in Twenty adult male Sprague-Dawley rats, which http://gut.bmj.com/ the presence of ethanol, and the chronic consump- initially weighed between 210 g and 310 g, were tion of ethanol is often associated with malnutrition maintained in single cages on a 12 hour light-dark and diarrhoea, but whether these are caused by cycle at 21 ±1°C room temperature.